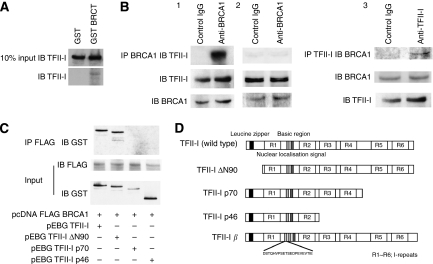
Figure 1.
In vivo and in vitro association between TFII-I and BRCA1, and mapping of the BRCT-interacting region of TFII-I. (A) Identification of the interaction between BRCT and TFII-I using GST-BRCT. Bacterially expressed GST fusion proteins immobilised on beads were used in in vitro pull-down assays. Nuclear extracts of HeLa cells were incubated with GST-BRCT. The beads were extensively washed, and followed by immunoblotting (IB) using anti-TFII-I antibodies. (B) The complex formation of TFII-I and BRCA1 in HeLa cells was analysed by co-immunoprecipitation (IP) with the antibodies to BRCA1 (epitope mapping at the carboxyl-terminus of BRCA1). The immunoblotting analysis using anti-TFII-I antibodies revealed the existence of TFII-I in cell lysate immunoprecipitates (Figure 1B, 1), which indicates that TFII-I physically associates with BRCA1 in living cells. Reciprocal immunoprecipitation analysis confirmed the association of TFII-I and BRCA1 (Figure 1B, 3). The whole-cell extracts of HCC1937 cells known to lack last BRCT domain were also immunoprecipitated with anti-BRCA1 antibodies (epitope mapping at the amino-terminus of BRCA1). The immunoblotting analysis revealed the absence of TFII-I in cell lysate immunoprecipitates (Figure 1B, 2), indicating the importance of BRCT as a binding surface of TFII-I. (C) Mapping of the BRCT-interaction region of TFII-I. COS7 cells were transfected with GST-tagged TFII-I (wild type, ΔN90, p70, and p46) and Flag-tagged BRCA1 expression vectors. Nuclear extracts of transfected COS7 cells were prepared and the complex formation of TFII-I and BRCA1 was analysed by IP with the anti-Flag M2 agarose beads, followed by IB using anti-GST antibodies. (D) A schematic diagram of the structure of TFII-I (wild type, ΔN90, p70, p46, and β isoform) is shown.