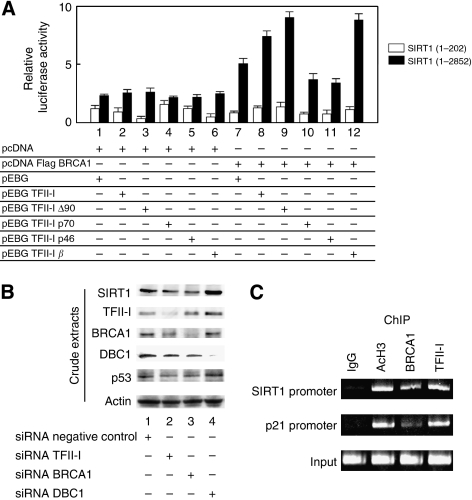
Figure 4.
TFII-I stimulates transcription by BRCA1 through its carboxyl-terminal domain. (A) Transient transfection assays were performed to examine the influence of TFII-I using an artificial luciferase reporter constructs. COS7 cells were transfected with the indicated combinations of mammalian expression plasmids. At 24 h after transfection, cells were harvested, and transfected whole-cell lysates were assayed for luciferase activity produced from the reporter plasmids. Full-length TFII-I and TFII-I ΔN90 showed specific upregulation of SIRT1 (1-2852)-luciferase activity mediated by BRCA1, while TFII-I p70 and TFII-I p46, lacking BRCT-interaction region, had no effect on SIRT1-luciferase activity. TFII-I showed no effect on SIRT1 (1-202)-luciferase activity that lack the binding domain of BRCA1. (B) The siRNA-mediated knockdown of BRCA1 decreased the expression of SIRT1. Knockdown of TFII-I also resulted in downregulation of SIRT1. Expression of BRCA1 and p53 was decreased by depletion of TFII-I. HeLa cells were transfected with indicated siRNA. At 48 h after transfection, cells were harvested and analysed by western blotting. (C) Chromatin immunoprecipitation assay was performed to confirm the recruitment of BRCA1 and TFII-I at the SIRT1 gene promoter and the p21 gene promoter. Both promoter regions are known to recruit BRCA1.