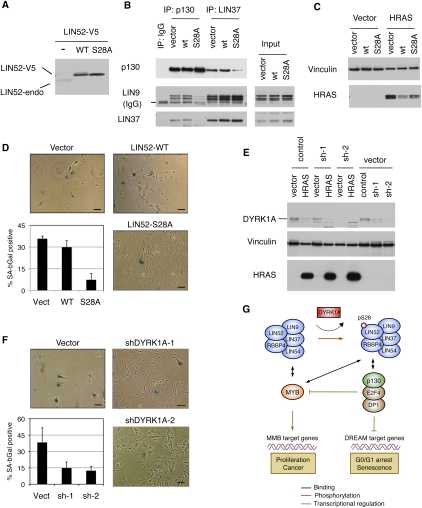
Figure 6.
DYRK1A and intact LIN52-S28A contribute to oncogenic Ras-induced senescence. (A) Western blot shows that overexpression of either the wild type or LIN52-S28A mutant in BJ-hTERT cells results in down-regulation of the endogenous LIN52 protein. (B) The LIN52-S28A mutant but not wild-type LIN52 disrupts the DREAM complex in BJ-hTERT fibroblasts, as shown by immunoprecipitation/Western blot assay. (C) The indicated BJ-hTERT cell lines were infected with retroviruses to express HRAS-G12V or empty vector, and the expression of HRAS-G12V was assayed by Western blot. Vinculin was used as a loading control. (D) BJ-hTERT cells expressing LIN52 alleles or vector were infected with HRAS-G12V and stained for SA-β-gal on day 14 post-infection. Representative images of the cells are shown as well as average values ± SD of three independent experiments, each counting >100 cells per condition. Two-tailed t-test: vector versus LIN52 wild type (LIN52-WT) and S28A mutant (LIN52-S28A), P = 0.24 and 0.01, respectively. Bar, 100 μM. (E) BJ-hTERT cells were transduced with either control or two different DYRK1A-specific shRNA lentiviruses, followed by infection with retrovirus encoding HRAS-G12V. Western blot shows the levels of DYRK1A and HRAS-G12V in these cells. Vinculin was used as a loading control. (F) Ras-induced senescence assay in BJ-hTERT cell lines treated with DYRK1A-shRNA. The cells were infected and processed as in D. Two-tailed t-test: vector versus shDYRK1A-1 and shDYRK1A-2, P = 0.09 and 0.05, respectively. Bar, 100 μM. (G) The model shows how DYRK1A promotes the DREAM complex assembly, G0/G1 arrest, and senescence. DYRK1A phosphorylation of the Ser 28 residue in the LIN52 subunit of the MuvB core promotes the DREAM complex assembly and repression of E2F target genes such as BMYB. MuvB core subunits are shaded in blue.