Figure 5.
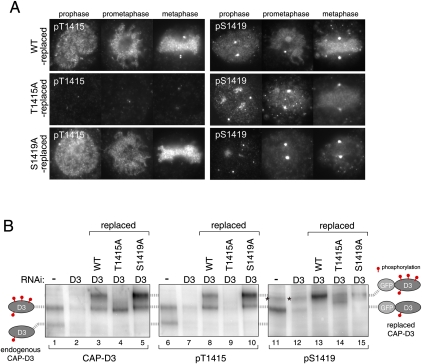
Hyperphosphorylation of CAP-D3 depends on CAP-D3-bound Plk1. (A) Reduced levels of Ser 1419 phosphorylation in T1415A-replaced cells. Cells in which endogenous CAP-D3 was replaced by either wild-type (top panels), T1415A (middle panels), or S1419A (bottom panels) forms of GFP-CAP-D3 were fixed and stained with pT1415 or pS1419 antibodies. Note that pS1419 antibodies cross-react with an unidentified epitope at centrosomes, seen as two marked dots that do not disappear after depletion of CAP-D3. Quantification of fluorescence intensities is summarized in Supplemental Figure 5, C and D. (B) Regulation of Thr 1415 and Ser 1419 phosphorylation. (First two lanes of each panel) Two species with different phosphorylation levels of endogenous CAP-D3 can be detected in mitotic cell extracts that diminish after CAP-D3 depletion, as depicted on the left side. (Last three lanes of each panel) Mitotic extracts from wild-type-replaced, T1415A-replaced or S1419A-replaced cells were analyzed. As replaced CAP-D3 proteins are tagged with GFP, they migrate slower than the endogenous proteins. The two corresponding phosphorylated forms of GFP-CAP-D3 are positioned on the right side. Asterisks mark nonspecific bands, which do not appear in immunopurified GFP-CAP-D3 samples (Supplemental Fig. 6A).