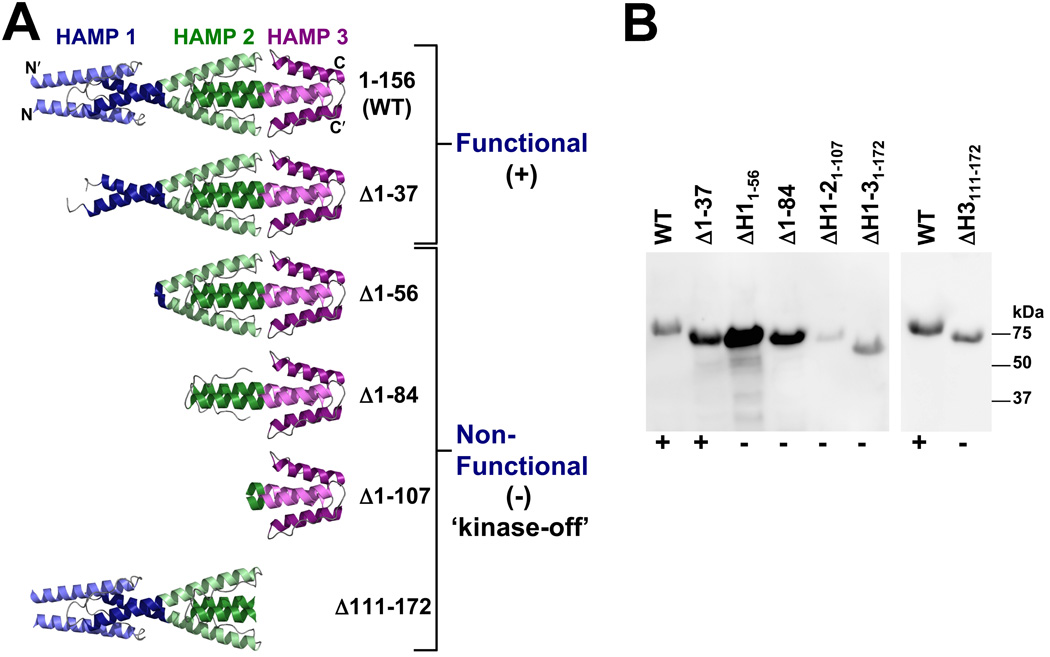
FIG. 5.
Influence of N-terminal HAMP domains 1, 2 and 3 on Aer-2 signaling. A. Structure of the N-terminal HAMP domains (Airola et al., 2010) and models of the truncation mutants that were tested for function in behavioral assays. The Δ1–37 mutant was the only truncated product that retained Aer-2 function in E. coli (+). The other truncation mutants all resulted in kinase-off phenotypes (−). See text for details. B. HisProbe Western blot showing size differences among the truncated Aer-2 products as well as variations in their steady-state accumulation levels in E. coli BT3388 after induction with 50 µM IPTG. Abbreviation: ΔH, ΔHAMP.