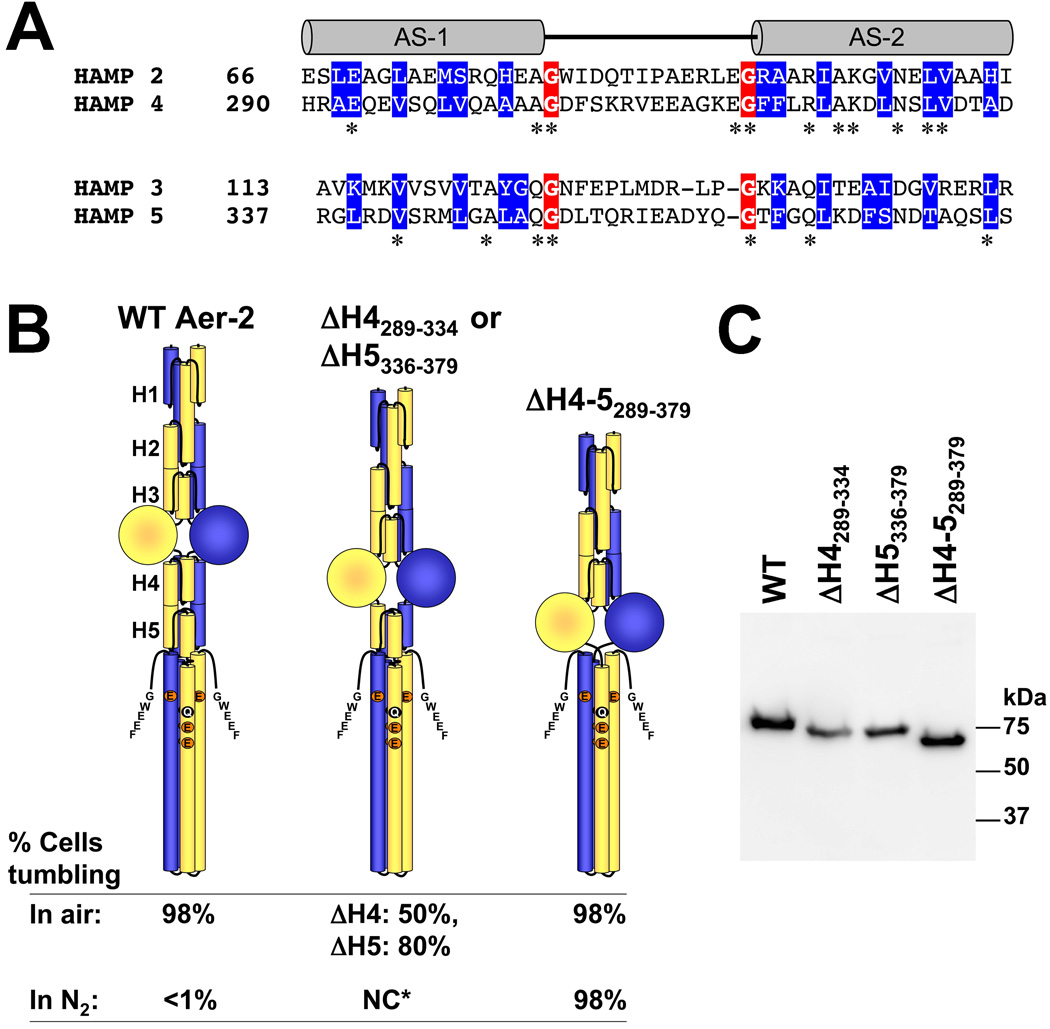
FIG. 6.
Influence of C-terminal HAMP domains 4 and 5 on Aer-2 signaling. A. Sequence alignment of di-HAMP 2–3 and di-HAMP 4–5. Connector-flanking glycine residues are shaded red, whereas buried core residues (based on the resolved structure of HAMP 2–3) are shaded blue. Stars indicate identical N- and C-terminal HAMP residues. B. HAMP 4 and 5 are required to maintain the kinase-off state. Aer-2 deleted for HAMP 4 and 5 (far right) exhibited maximal kinase-on activity in air (tumbling in E. coli BT3388), like WT (far left). However, unlike WT, the ΔHAMP 4–5 mutant remained kinase-on (no change, NC) in N2 (in the absence of a diatomic oxy-gas). Mutant proteins lacking either HAMP 4 or HAMP 5 exhibited intermediate kinase-on activities (center figure). The HAMP 5 deletion began at residue 336 because residue 335 forms part of HAMP 4 and HAMP 5. C. HisProbe Western blot showing size differences among the internally truncated Aer-2 products and variations in their steady-state accumulation levels in E. coli BT3388 after induction with 50 µM IPTG. Abbreviations: AS, amphipathic sequence; H, HAMP; NC*, no change for either mutant.