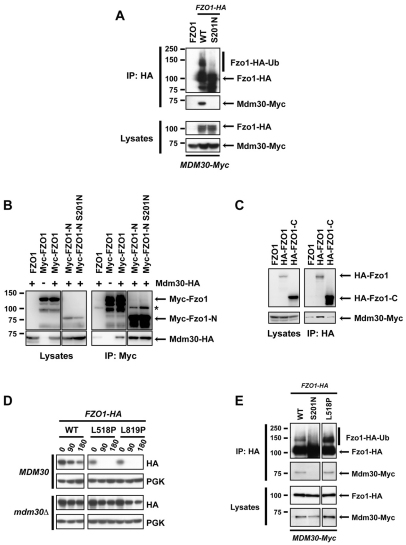
Fig. 2.
Accessibility of Mdm30 to its N-terminal Fzo1 binding site is controlled by the Fzo1 C-terminal region in a GTPase domain-dependent manner. Experiments were performed in DCY538 background cells. Myc–Fzo1-N and HA–Fzo1-C are both N-terminally tagged in B and C. N-terminally instead of C-terminally tagged versions of full-length Fzo1 were therefore used for consistency. (A) fzo1Δ mdm30Δ cells expressing untagged Fzo1 (FZO1), Fzo1–HA (WT) or Fzo1-S201N–HA (S201N) together with Mdm30 epitope-tagged at its C-terminus (Mdm30–Myc) were subjected to co-immunoprecipitation with anti-HA followed by immunoblotting as indicated and developed using ECL. Top panels, immunoprecipitates; bottom panels, lysates (10% input of IP). (B) Co-immunoprecipitations were performed as in A with strains that either express (+) or do not express (−) C-terminal HA-tagged Mdm30 and transformed with a plasmid expressing either WT or GTPase mutant (S201N) forms of either full-length or truncated (Myc–Fzo1-N, aa 1–415) Fzo1 bearing nine Myc epitope tags at their N-termini. Right panels, immunoprecipitates; left panels, lysates (10% input of IP). Incomplete reduction of a small amount of IgG heavy chain dimers that is variably observed is indicated (*). (C) Co-immunoprecipitations with anti-HA were performed as in A with strains co-expressing indicated N-terminal 6-HA-tagged forms of full-length or truncated (HA–Fzo1-C; aa 416–855) Fzo1 and C-terminal Myc-tagged Mdm30. Right panels, immunoprecipitates; left panels, lysates (10% input of IP). (D) Cycloheximide (CHX) chase performed in fzo1Δ (Top) and fzo1Δ mdm30Δ (bottom) strains expressing indicated versions of C-terminal HA-tagged forms of either WT Fzo1 or Fzo1 mutated in either its HR1 (L518P) or HR2 (L819P) domain. (E) fzo1Δ mdm30Δ cells expressing WT, S201N or L518P forms of Fzo1-HA together with Mdm30-Myc were subjected to co-IP with anti-HA followed by immunoblotting with anti-HA or anti-Myc and detection using ECL. Top two panels, immunoprecipitates; bottom two panels, lysates (10% input of IP).