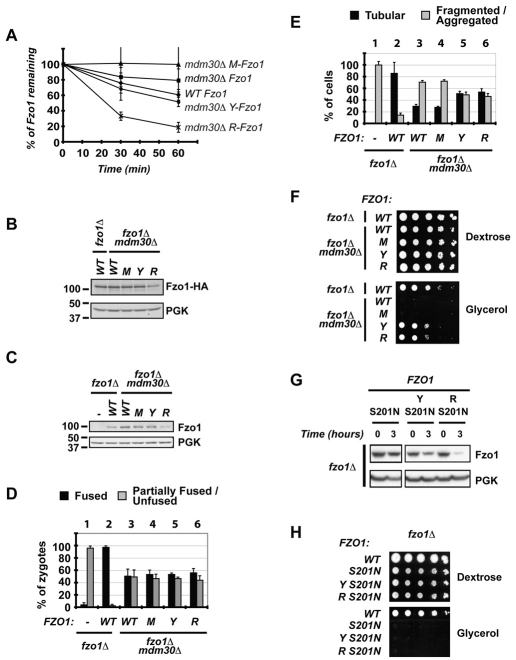
Fig. 4.
Degradation of Fzo1 by the N-end-rule pathway partially bypasses the requirement for Mdm30 in mitochondrial function. All experiments were performed in fzo1Δ or fzo1Δ mdm30Δ cells (DCY538 background) expressing untagged versions of Fzo1 unless indicated: (−), empty vector; (WT), Fzo1; (M), Ub–M-Fzo1; (Y), Ub–Y-Fzo1; (R), Ub–R-Fzo1. Error bars are the s.d. from three independent experiments. (A) Analysis of degradation of indicated forms of Fzo1–HA by [35S]Met metabolic labeling. (B) Immunoblot of steady state Fzo1–HA from whole cell extracts using anti-HA. PGK was used as a control for equal loading. (C) Immunoblot of steady state Fzo1 from whole cell extracts using anti-Fzo1. PGK was used as a control for equal loading. (D) In vivo mitochondrial fusion assay. Percentage of zygotes (derived from mating of DCY538 and JSY2294 strains expressing indicated versions of Fzo1) with fused or partially fused/unfused mitochondria. (E) Percentage of cells with tubular or fragmented/aggregated mitochondria. (F) Serial dilutions of indicated strains on dextrose or glycerol medium. (G) Cycloheximide (CHX) chase performed in fzo1Δ strains expressing S201N mutants of Fzo1 (untagged) fused, or not, to indicated N-end rule degrons (Y, R). Immunoblotting was for Fzo1 or PGK. (H) Serial dilutions of strains expressing WT untagged Fzo1 or the S201N mutants (as in C) on dextrose or glycerol medium.