Abstract
The structure of the environment surrounding signal emission produces different patterns of degradation and attenuation. The expected adjustment of calls to ensure signal transmission in an environment was formalized in the acoustic adaptation hypothesis. Within this framework, most studies considered anuran calls as fixed attributes determined by local adaptations. However, variability in vocalizations as a product of phenotypic expression has also been reported. Empirical evidence supporting the association between environment and call structure has been inconsistent, particularly in anurans. Here, we identify a plausible causal structure connecting environment, individual attributes, and temporal and spectral adjustments as direct or indirect determinants of the observed variation in call attributes of the frog Hypsiboas pulchellus. For that purpose, we recorded the calls of 40 males in the field, together with vegetation density and other environmental descriptors of the calling site. Path analysis revealed a strong effect of habitat structure on the temporal parameters of the call, and an effect of site temperature conditioning the size of organisms calling at each site and thus indirectly affecting the dominant frequency of the call. Experimental habitat modification with a styrofoam enclosure yielded results consistent with field observations, highlighting the potential role of call flexibility on detected call patterns. Both, experimental and correlative results indicate the need to incorporate the so far poorly considered role of phenotypic plasticity in the complex connection between environmental structure and individual call attributes.
Keywords: acoustic adaptation hypothesis, call adjustment, Hypsiboas pulchellus, local adaptation, phenotypic plasticity, scale
INTRODUCTION
The transfer and sharing of information are a central component of the interaction between individuals (Schwartz 2001). To be effective, a signal should be detected and discriminated by the receiver (Schwartz 2001). However, the environment can induce modifications that degrade signal structure (Wiley and Richards 1978; Richards and Wiley1980; Forrest 1994; Bradbury and Vehrencamp 1998). In this sense, in most natural situations signals do not reach receivers exactly as they were emitted due to the effect of interference with physical objects or with other signals that use the same channel simultaneously (Narins and Zelick 1988; Forrest 1994). Acoustic signals may be masked by both conspecific and heterospecific individuals, whereas call modulation during propagation is often associated with local habitat structure and climatic factors (Wiley and Richards 1978; Richards and Wiley 1980; Wiley 1991; Bradbury and Vehrencamp 1998).
The acoustic adaptation hypothesis (AAH) proposes that selection dependent on habitat structure has molded the evolution of the basic properties of calls (Morton 1975; Hansen 1979). This hypothesis predicts that calls with low frequencies, narrow bandwidths, low-frequency modulations, and long elements and interelement intervals should prevail in environments with high attenuation (Morton 1975; Wiley and Richards 1978). These predictions have been tested mainly in birds but also in other model organisms (Ey and Fischer 2009), by contrasting calls among populations of the same or different species (e.g., Hunter and Krebs 1979; Shy and Morton 1986; Wiley 1991; Badyaev and Leaf 1997; Saunders and Slotow 2004). It should be highlighted that variation in call phenotypes has traditionally been seen as the fixed outcome of microevolutionary processes (Piersma and Drent 2003; West-Eberhard 2003). However, selection could have favored plastic phenotypes instead of fixed ones (Scheiner 1993; Via 1993; West-Eberhard 2003).
It has been shown that some species are capable of adjusting acoustic emissions in response to habitat characteristics (Lardner and bin Lakim 2002; Slabbekoorn and Peet 2003; Patricelli and Blickley 2006). These adjustments are under the organism's behavioral regulation and are cases of phenotypic flexibility (Piersma and Drent 2003; Dingemanse et al. 2010). Flexibility is a form of phenotypic plasticity, which is the expression of environment-dependent phenotypes (Piersma and Drent 2003; Dingemanse et al. 2010). Acoustic communication typically involves a variety of environmental contexts for signal propagation, with a potential advantage for flexible phenotypes. The bulk of research addressing phenotypic flexibility in acoustic communication comes from primates (see Jones 2005) and birds (Slabbekoorn and Peet 2003; Patricelli and Blickley 2006). Studies in amphibians are scarce and have focused on socially induced plasticity (Lucas and Howard 1995; Lucas et al. 1996; Bee et al. 2000; Bee and Bowling 2002).
Acoustic signals play fundamental roles in anuran communication, by facilitating social interactions, mediating reproduction, and determining vulnerability or risk of predation (Gerhardt 1994; Hödl and Amézquita 2001; Page and Ryan 2005; Bernal et al. 2007). Given their central role in reproduction, advertisement calls are subject to strong sexual selection (Ryan 2001; Gerhardt and Huber 2002). However, only a few studies have explored the capability of individuals to evaluate the signal propagation environment and adjust their vocalizations. Lardner and bin Lakim (2002) reported what appears to be the only evidence of a frog able to evaluate the acoustic properties of its physical environment and concurrently adjust its calls.
Hypsiboas pulchellus (Anura: Hylidae) occupies a wide array of calling environments representing an exceptional model with which to evaluate the match between call attributes and environmental structure. In this study, we estimate a plausible causal structure directly and indirectly connecting environment, male size, and call attributes and experimentally demonstrate the ability of males to produce short-term adjustments in their calls.
MATERIALS AND METHODS
Hypsiboas pulchellus is a medium-sized frog (snout-vent length: 30–46 mm; mass: 1.8–4.7 g). It occurs in southern South America, specifically in Uruguay, southern Brazil, central and northeastern Argentina, and southern Paraguay. Male choruses can be found at throughout the year, without marked seasonality (Canavero et al. 2008). Males call from ponds and forested streams and from a range of perch heights, which exhibit markedly different sound propagation properties (Mitchell and Miller 1991; Nemeth et al. 2001).
The advertisement call of H. pulchellus was described by Basso NG and Basso G (1987). It is a simple call consisting of 2 notes, here designated as note 1 and 2, respectively. The call is monophasic, not presenting modulations in frequency (Littlejohn 2001). For this species, in both notes 1 and 2, the harmonic containing the greatest amount of energy (dominant frequency) coincides with the first harmonic (fundamental frequency).
Fieldwork was carried out on farmland located in Paraje Costa Pando, Canelones, Uruguay (lat 34°34′21″S; long 55°56′40″W). This area includes a rich array of microhabitats in which amphibians are found calling. Advertisement calls were recorded on chrome tape cassettes using an analog recorder (SME Marantz PMD 222) and a directional microphone (Sennheiser ME66/K6). Calls were digitized and analyzed using an Edirol/Roland UA-1EX USB interface in Audacity 1.2.6 (freeware version). Both spectrograms and oscillograms were generated for each call, extracting the dominant frequency of each note, call rate, note duration, internote interval, and call duration. These are robust descriptors of species' advertisement calls among anuran species (Gerhardt 1991; Gerhardt and Huber 2002).
The methodological approach was done in 2 steps. First, the association between physical environment and individual and call attributes was explored. Through path analysis, we identified a plausible causal structure beyond the observed associations. Then, we used experimental evidence to test for a putative role of phenotypic plasticity on the observed patterns.
Association between call attributes and environment
Calling males were recorded at a distance of <50 cm for at least 2 min, or one calling bout. After each recording, males were captured and weighed with a precision spring scale to the nearest 0.1 g (Pesola) and measured (snout-vent length) with a digital caliper (Mitutoyo) to the nearest 0.1 mm. Calling sites were marked with a numbered stake to allow vegetation sampling the next day. Habitat structure characterization was carried out through measurement of vegetation density (% cover) in 25 × 25 cm quadrats. Vegetation was measured at ground level where a frog had been sitting or perching and at heights of 0.5 and 1 m. These measures were repeated at distances of 1 and 2 m in 4 directions, resulting in a total of 27 quadrats measured per frog. Site temperature was also measured to the nearest 0.5 °C. Another variable, percentage of water in the environment, was derived from the measurement of vegetation density. Because the habitat consisted mostly of vegetation covering the water surface, all quadrats that contained only water were summed for each individual and calculated as a percentage over the measures taken at ground level for each calling site.
Exploration of causal structure beyond associations
The connections between call attributes, environmental conditions, and individuals' body mass were evaluated with path analysis (Shipley 2002). This analysis provides a robust technique with which to evaluate ecological and evolutionary hypotheses from observed associations among variables (Nespolo et al. 2003; Kline 2005; Arhonditsis et al. 2006). Hypotheses about causal relationships are represented by a path diagram and evaluated statistically (Shipley 2002). This evaluation requires at least 5 observations per parameter (Shipley 2002). In order to minimize the number of parameters estimated in the path model and to account for colinearity among variables, principal components analysis (PCA) was used to summarize both vegetation and call variables (Shipley 2002; Kline 2005; Carrascal et al. 2009). Additional requirements of the analysis—linear associations and additivity—were met by the data (Shipley 2002; Kline 2005). Path analysis is based in the fact that once a causal structure among variables is suggested, the complete set of expected variances–covariances is completely determined (Shipley 2000). The technique is based in the statistical contrast between the observed and expected variance–covariance matrix from the path model. Therefore, a path model represents a reliable estimation of the true causal structure when the statistical contrast is not significant (see Shipley 2002; Pugesek et al. 2003). For each single path, the associated coefficients and their significance are interpreted in the same way as coefficients of multiple regressions (Shipley 2000).
The analysis of the causal structure involved 3 stages (following Kline 2005). We first reduced the dimension of the system using principal components analysis (PCA). Then, to explore environmental determinants of call attributes, we performed 2 generalized additive model (GAM) analyses, using factors extracted from call attributes as dependent variables and the other variables as potential independent ones. Finally, we proposed a model and generated the covariance matrix for path analysis. Both structural equation model and GAM analyses were performed with R (R Development Core Team 2009), using the packages “sem” (Fox and Kramer 2009) and “gam” (Hastie 2009).
Evaluation of call flexibility
The ability of frogs to modulate the structure of their calls in response to variation in their physical environment was evaluated by altering the environment of each calling male. To alter the local acoustic environment, we used an open styrofoam enclosure (dimensions 0.5 × 0.5 × 0.5 m; thickness: 10 mm), without top or bottom. Eight calling males were recorded in free-field conditions. The enclosure was then placed on the substrate around each frog, and once calling was resumed, the animals' calls were rerecorded, with the microphone placed inside the enclosure. These data were analyzed performing a paired t-test for the whole assemblage of individuals (Sokal and Rohlf 1995). In this analysis, the mean values of each call parameter before and during the treatment were paired for each individual. In order to visualize the response of individuals to the treatment, a PCA was performed considering those temporal variables of the advertisement call, which were significant in the paired t-test (Sokal and Rohlf 1995).
It is important to highlight that given the dimensions of the enclosure and the length of call components, the existence of echoes in the recorded calls was negligible. Nevertheless, the potential existence of reverberations or other degradation effects related to treatment were evaluated. A control experiment was performed in which we rerecorded playbacks broadcast within the styrofoam enclosure and compared these recordings with the original ones. The calls of 7 of the males that were tested in the enclosure were broadcast from within the enclosure and rerecorded in the same way as experimental males. These new recordings were analyzed as previously described.
RESULTS
Causal connection between environment and call structure
PCA for vegetation structure revealed 3 factors that accounted for 84.8% of the variation (Table 1). The first factor had higher loadings for vegetation at the site of the frog and at a distance of 1 m from it, and the second factor had higher loadings for vegetation 2 m from the frog, above ground (Table 1). PCA for call variables yielded 2 factors that accounted for 82.5% of the observed variation (Table 2). Factor 1 represented those variables associated with the temporal structure of the advertisement call, whereas factor 2 represented the spectral variables (i.e., dominant frequency of the first and second note, Table 2).
Table 1.
Factor loadings and percentage of variance explained for the vegetation PCA
| Factor 1 | Factor 2 | Factor 3 | |
| h0, d0 | −0.65 | −0.59 | −0.17 |
| h0, d1 | −0.75 | −0.32 | −0.50 |
| h0, d2 | −0.65 | −0.36 | −0.47 |
| h1, d0 | 0.72 | 0.32 | −0.40 |
| h1, d1 | 0.88 | 0.10 | −0.38 |
| h1, d2 | 0.37 | −0.76 | 0.39 |
| h2, d0 | 0.47 | −0.64 | −0.32 |
| h2, d1 | 0.79 | −0.39 | −0.39 |
| h2, d2 | 0.36 | −0.77 | 0.41 |
| % Total variance | 42.5 | 27.6 | 15.2 |
| Cumulative variance | 42.5 | 69.6 | 84.8 |
All variables are percentage cover, and for each variable, the first term indicates height (h0, ground level; h1, 0.5 cm above ground level; and h2, 1 m above ground level), and the second term indicates distance from calling site (d0, site of frog calling; d1, 1 m from the frog; and d2, 2 m from the frog).
Table 2.
Factor loadings and percentage (single and cumulative) of variance explained for the call PCA
| Factor 1 | Factor 2 | |
| DF1 | −0.02 | 0.99 |
| DF2 | 0.02 | 0.99 |
| DN1 | 0.58 | 0.18 |
| DN2 | 0.95 | −0.03 |
| DC | 0.99 | −0.02 |
| INI | 0.86 | −0.06 |
| % Total variance | 49.4 | 33.1 |
| Cumulative variance | 49.4 | 82.5 |
DF1, dominant frequency of note 1; DF2, dominant frequency of note 2; DN1, duration of note 1; and DN2, duration of note 2; CD, call duration; and INI, internote interval.
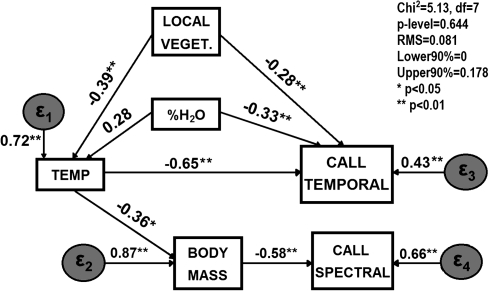
These factors were taken as new variables, which together with body weight, snout-vent length, percentage of water in the environment, and temperature, were taken into account in the next stage before building the model. GAM analyses suggested a main effect of local vegetation (represented in the first factor of the PCA), temperature, and proportion of water in the environment as determinants of temporal variables (first factor in call PCA). In addition, spectral variables were only related to the size of calling males. From these results, we constructed a path model, which in addition indicated the existence of an effect of local vegetation and proportion of water on local temperature, which also affected the size of calling males (see Figure 1). This model was robust and largely congruent with observations (; p = 0.64). All paths were significant at the 0.05 level, with the exception of the one linking “% water” and temperature, which was marginally significant (see Figure 1).
Figure 1.
Path analysis representing the causal model that best explained the relationship among abiotic and call variables. Path coefficients are indicated for each path (link) between variables. local veget. = local vegetation, corresponding to the first axis of a PCA for vegetation density; %H2O = percentage of open water in the calling site; temp = temperature; and call temporal and call spectral = temporal and spectral call parameters, corresponding to the first and second PCA axes, respectively. Epsilons (ϵ) represent variances unexplained by the model. RMS = root mean square error.
In summary, the path diagram indicates the existence of 2 main determinants of call attributes. On the one hand, environmental conditions consistently affect temporal attributes, and on the other, individual attributes (body mass) affect the spectral structure of calls. However, it should be highlighted that environmental conditions, through their effect on the size of calling males, could be indirectly affecting the spectral structure of calls. Alternative models directly connecting environmental variables to spectral attributes were evaluated. However, these models did not predict a covariance structure consistent with the observed data (Shipley 2002).
Experimental evidence for call flexibility
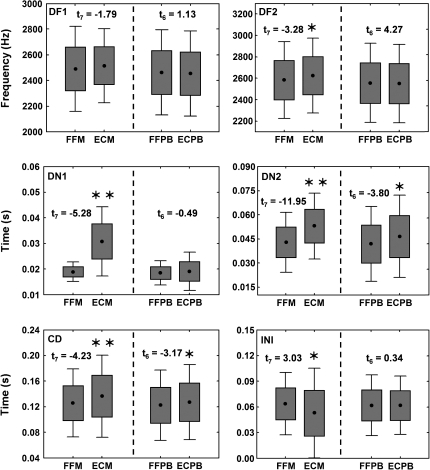
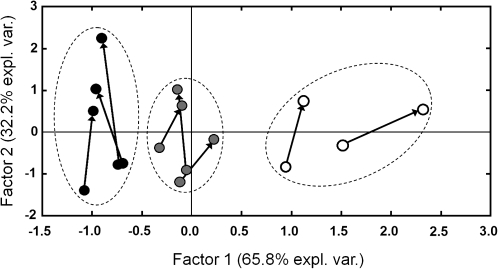
Calling males both in free-field conditions and inside the styrofoam enclosure showed a pattern congruent with an adjustment to their physical calling environment. The point of departure for the different call attributes for spectral and temporal parameters did not differ between free field and enclosure recordings. In this sense, dominant frequency of notes 1 (t7 = 2.02) and 2 (t7 = 0.74), duration of notes 1 (t7 = −1.41) and 2 (t7 = −1.86), and total call duration (t7 = 1.01) were all nonsignificantly different at the 0.05 level. The exception was the duration of internote interval (t7 = −2.62; p = 0.035). Contrasting with these nonsignificant results, in paired comparisons across all individuals for the full call section, temporal variables, and dominant frequency of the second note exhibited significant differences (see Figure 2). All temporal variables showed a consistent trend, with duration of the first and second note and total call duration being significantly longer when emitted from within the styrofoam enclosure, whereas internote interval was significantly shorter (Figure 2). PCA of the temporal variables revealed a consistent response among all frogs in the enclosure experiment (Figure 3). For this analysis, the first axis represents all temporal variables with the exception of duration of the first note, which is represented by the second axis.
Figure 2.
Effect of the styrofoam enclosure on call structure. Boxplots show the result of Student's t-test for paired comparisons. FFM, male free-field recording; ECM, males in the enclosure; FFPB, free-field playback; and ECPB, playback in the enclosure. Individual graphs represent call parameters: DF1, dominant frequency of note 1; DF2, dominant frequency of note 2; DN1, duration of note 1; DN2, duration of note 2; CD, call duration; and INI, internote interval. * for p < 0.05, ** for p < 0.01.
Figure 3.
Scatterplot showing the trajectory of temporal variables for males tested with the styrofoam enclosure. Each starting dot corresponds to a male calling in natural conditions, and the arrowheads show the final position, with that male calling from inside the enclosure. Factor 1 and Factor 2 correspond to the first and second axes, respectively, obtained from the PCA performed for temporal variables. Factor 1 represents all temporal variables with the exception of note 1 duration, which is represented by the second factor. Black circles correspond to males calling in a pond on the night of 26 October 2008; gray circles correspond to males calling in a pond on the night of 5 January 2009; and white circles correspond to males calling in a section of forested stream on the night of 27 October 2008.
Most call parameters were not affected by the treatment, with the exception of total call duration and duration of note 2. However, for duration of note 2, the magnitude of the change observed when the playback was broadcast from the enclosure was smaller than the change produced by the frogs themselves when tested in the enclosure. Most important, it should be highlighted that note 1 duration, the parameter that was most consistently and significantly modulated by frogs was not affected by the experimental procedure (see Figure 2).
DISCUSSION
Intraspecific variation in calls among environments has been explained through differences in the identity of emitters (see Ey and Fischer 2009). However, we show the potential existence of a mechanism previously overlooked and operating at a finer scale. The causal structure identified through path analysis, combined with experimental evidence of males' ability to fine-tune their calls, suggests a role of phenotypic flexibility in the adjustment of calls to the environment.
Males of H. pulchellus have the potential to adjust their calls in response to their local environment. The fact that call attributes were not significantly different at the start of the experiment between free field and enclosure records but differed significantly for the complete call indicates that some process of modulation is involved. Determinants of individuals' call decisions have been poorly considered. However, size-dependent energetic reserves, the effect of temperature on metabolic activity and their effects on the potential to allocate energy to reproductive effort are the main mechanisms involved (McLister 2001; McNab 2002; Kiss et al. 2009). The existence of flexibility in call attributes draws attention to other underlying mechanisms. This kind of flexibility presumes the existence of a feedback response, involving a cognitive process between signal emission and detection of its potential attenuation and/or degradation (Kelley 2004; Eliades and Wang 2008). This phenomenon has been reported mainly in primates (Eliades and Wang 2008). There exists only one report—for only one individual of one species—for amphibians (Lardner and bin Lakim 2002). Although there are studies that address bird signal variation in response to ambient noise (see for examples Slabbekoorn and Peet 2003; Patricelli and Blickley 2006), this evidence is based on different individuals under different noise levels. In this sense, experimental and correlative approaches, as the ones here introduced, could improve the understanding of call modulation in other taxa, and its effects in ecological and evolutionary processes. Also, the present contribution suggests that individuals can use acoustic feedback of their own calls to match propagation properties of their environment in real-time. Yet, there is heterogeneity in the strength of the response and its direction (i.e., modulation upwards or downwards). Exploring the underlying causes of such heterogeneous response now appears as an interesting topic for future research.
Flexibility involves a more complex scenario for signal transmission than previously thought (Endler and Basolo 1998; Foster 1999; Boul et al. 2007). Call flexibility in response to the acoustic properties of the environment affects signal transmission and female attraction (Endler 1992). This implies a potential connection between flexible phenotypes and sexual selection and in how environmentally driven changes in mating behavior can set the context through which selection drives evolutionary changes (Price 2006). In this sense, this article opens the door to the analysis of these phenomena within the framework of behavioral reaction norms (Dingemanse et al. 2010). In addition, our results also support classic interpretations where different calls are attributed to the fact that individuals are different rather than individuals displaying flexible calls (Zimmerman 1983; Bosch and De la Riva 2004; reviewed in Ey and Fischer 2009). Specifically, spectral attributes were related to body size of calling males (Figure 1). As a consequence, both phenotypic plasticity and fixed attributes appear as important determinants of the observed call structure.
The process determining the association between calls and environment could change at different scales of observation, accounting for the mismatch between habitat type and call structure reported elsewhere (Feng and Schul 2007). The association between habitat and call attributes has been focused mainly at the macrohabitat scale, with few exceptions (Bosch and De la Riva 2004; Yao and Lin 2004). This scale of analysis is congruent with the view of call variation as the result of local adaptations. Regarding amphibians, the limited information available shows little agreement on the potential of the AAH to account for observed call patterns (Bosch and De la Riva 2004). This could be due to inconsistencies between the scale of observation and the scale at which mechanisms operate when call flexibility is involved (Zimmerman 1983; Penna and Solís 1998; Kime et al. 2000; Bosch and De la Riva 2004).
Final remarks
The match between call structure and environment has wide implications in organism performance and evolution. Although most natural variation in calling structure has been studied assuming it as a fixed attribute, our results suggest that call flexibility is also involved. The explicit consideration of a complex causal structure connecting environment, individual attributes, and call structure, allowed to jointly analyze the connection between components that have traditionally been separately considered. An additional implication of our results is that the temporal and spatial scale of changes in calls could be much smaller than previously considered. Thus, our study attempts to advance on the connection between call attributes and environment, highlighting the potential role of call flexibility and unraveling the association between environment, individual traits, and call structure.
FUNDING
L.Z. received financial support from Programa de Desarrollo de las Ciencias Básicas (Universidad de la República, Uruguay) and from the Agencia Nacional de Investigación e Innovación, Uruguay. Idea Wild donated to L.Z. all the recording equipment used throughout this study. The Acoustical Society of America through its Committee on International Research and Education awarded a Student Award to L.Z., which was used to partially support the research. M.A. thanks Fondo Clemente Estable, grant number 05076, and FONDAP-FONDECYT 1501-0001 for their support, and P.M.N. gratefully acknowledges National Institute of Health grant DC00222 for its support.
Supplementary Material
Acknowledgments
We wish to thank the Editor and 2 anonymous reviewers for their comments and suggestions, which we believe have contributed to a notable improvement of this manuscript. Our gratefulness goes to Gabriel Laufer, Matías Zarucki, Juan Manuel Barreneche, Luis Orlando, Mauro Berazategui, and Alejandro Duarte for field assistance. All members of the Jolgory Lab significantly contributed with lucid discussion.
References
- Arhonditsis GB, Stow CA, Steinberg LJ, Kenney MA, Lathrop RC, McBride SJ, Reckhow KH. Exploring ecological patterns with structural equation modeling and Bayesian analysis. Ecol Model. 2006;192:385–409. [Google Scholar]
- Badyaev AV, Leaf ES. Habitat associations of song characteristics in Phylloscopus and Hippolais warblers. Auk. 1997;114:40–46. [Google Scholar]
- Basso NG, Basso G. Análisis acústico del canto nupcial de Hyla pulchella pulchella Dumeril & Bibron 1841 (Anura: Hylidae) An Mus Hist Nat Valparaíso. 1987;18:109–114. [Google Scholar]
- Bee MA, Bowling AC. Socially mediated pitch alteration byterritorial male bullfrogs, Rana catesbeiana. J Herpetol. 2002;36:140–143. [Google Scholar]
- Bee MA, Perrill SA, Owen PC. Male green frogs lower the pitch of acoustic signals in defense of territories: a possible dishonest signal of size? Behav Ecol. 2000;11:169–177. [Google Scholar]
- Bernal XE, Page RA, Rand AS, Ryan MJ. Cues for eavesdroppers: do frog calls indicate prey density and quality? Am Nat. 2007;169:409–415. doi: 10.1086/510729. [DOI] [PubMed] [Google Scholar]
- Bosch J, De la Riva I. Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. Can J Zool. 2004;82:880–888. [Google Scholar]
- Boul KE, Chris Funk W, Darst CR, Cannatella DC, Ryan MJ. Sexual selection drives speciation in an Amazonian frog. Proc R Soc B Biol Sci. 2007;274:399–406. doi: 10.1098/rspb.2006.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland (MA): Sinauer Associates; 1998. [Google Scholar]
- Canavero A, Arim M, Naya DE, Camargo A, da Rosa I, Maneyro R. Calling activity patterns in an anuran assemblage: the role of seasonal trends and weather determinants. NorthWest J Zool. 2008;4:29–41. [Google Scholar]
- Carrascal LM, Galván I, Gordo O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos. 2009;118:681–690. [Google Scholar]
- Dingemanse NJ, Kazem A, Réale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Endler JA. Signals, signal conditions, and the direction of evolution. Am Nat. 1992;139:s125–s153. [Google Scholar]
- Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Ey E, Fischer J. The “acoustic adaptation hypothesis”—a review of the evidence from birds, anurans and mammals. Bioacoustics. 2009;19:21–48. [Google Scholar]
- Feng AS, Schul J. Sound processing in real-world environments. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and sound communication in amphibians. Springer handbook of auditory research. New York: Springer-Verlag; 2007. pp. 323–350. [Google Scholar]
- Forrest TG. From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool. 1994;34:644–654. [Google Scholar]
- Foster SA. The geography of behaviour: an evolutionary perspective. Trends Ecol Evol. 1999;14:190–195. doi: 10.1016/s0169-5347(98)01577-8. [DOI] [PubMed] [Google Scholar]
- Fox J, Kramer A. sem: structural equation models. R package version 0.9-17. 2009. [cited 2011 January 24]. Available from: http://CRAN.R-project.org/package=sem. [Google Scholar]
- Gerhardt HC. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav. 1991;42:615–635. [Google Scholar]
- Gerhardt HC. The evolution of vocalization in frogs and toads. Annu Rev Ecol Syst. 1994;25:293–324. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago (IL): The University of Chicago Press; 2002. [Google Scholar]
- Hansen P. Vocal learning: its role in adapting sound structures to long-distance propagation, and a hypothesis on its evolution. Anim Behav. 1979;27:1270–1271. [Google Scholar]
- Hastie T. gam: generalized additive models. R package version 1.01. 2009. [cited 2011 January 24]. Available from: http://CRAN.R-project.org/package=gam. [Google Scholar]
- Hödl W, Amézquita A. Visual signaling in anuran amphibians. In: Ryan MJ, editor. Anuran communication. Washington (DC): Smithsonian Institution Press; 2001. pp. 183–204. [Google Scholar]
- Hunter ML, Krebs JR. Geographical variation in the song of the great tit (Parus major) in relation to ecological factors. J Anim Ecol. 1979;48:759–785. [Google Scholar]
- Jones CB. Behavioral flexibility in primates: causes and consequences. New York: Springer; 2005. [Google Scholar]
- Kelley DB. Vocal communication in frogs. Curr Opin Neurobiol. 2004;14:751–757. doi: 10.1016/j.conb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kime NM, Turner WR, Ryan MJ. The transmission of advertisement calls in Central American frogs. Behav Ecol. 2000;11:71–83. [Google Scholar]
- Kiss AC, de Carvalho JE, Navas CA, Gomes FR. Seasonal metabolic changes in a year-round reproductively active subtropical tree-frog (Hypsiboas prasinus) Comp Biochem Physiol A. 2009;152:182–188. doi: 10.1016/j.cbpa.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York: The Guilford Press; 2005. [Google Scholar]
- Lardner B, bin Lakim M. Tree-hole frogs exploit resonance effects. Nature. 2002;420:475. doi: 10.1038/420475a. [DOI] [PubMed] [Google Scholar]
- Littlejohn MJ. Patterns of differentiation in temporal properties of acoustic signals of anurans. In: Ryan MJ, editor. Anuran communication. Washington DC: Smithsonian Institution Press; 2001. pp. 102–120. [Google Scholar]
- Lucas JR, Howard RD. On alternative reproductive tactics in anurans: dynamic games with density and frequency dependence. Am Nat. 1995;146:365–397. [Google Scholar]
- Lucas JR, Howard RD, Palmer JG. Callers and satellites: chorus behaviour in anurans as a stochastic dynamic game. Anim Behav. 1996;51:501–518. [Google Scholar]
- McLister JD. Physical factors affecting the cost and efficiency of sound production in the treefrog Hyla versicolor. J Exp Biol. 2001;204:69–80. doi: 10.1242/jeb.204.1.69. [DOI] [PubMed] [Google Scholar]
- McNab BK. The physiological ecology of vertebrates: a view from energetics. Ithaca (NY): Cornell University Press; 2002. [Google Scholar]
- Mitchell SL, Miller GL. Intermale spacing and calling site characteristics in a southern Mississippi chorus of Hyla cinerea. Copeia. 1991;1991:521–524. [Google Scholar]
- Morton ES. Ecological sources of selection on avian sounds. Am Nat. 1975;109:17–34. [Google Scholar]
- Narins PM, Zelick R. The effects of noise on auditory processing and behavior in amphibians. In: Fritzsch B, Ryan MJ, Wilczynski W, editors. The evolution of the amphibian auditory system. New York: John Wiley; 1988. pp. 511–536. [Google Scholar]
- Nemeth E, Winkler H, Dabelsteen T. Differential degradation of antbird songs in a Neotropical rainforest: adaptation to perch height? J Acoust Soc Am. 2001;110:3263–3274. doi: 10.1121/1.1420385. [DOI] [PubMed] [Google Scholar]
- Nespolo RF, Arim M, Bozinovic F. Body size as a latent variable in a structural equation model: thermal acclimation and energetics of the leaf-eared mouse. J Exp Biol. 2003;206:2145–2157. doi: 10.1242/jeb.00396. [DOI] [PubMed] [Google Scholar]
- Page RA, Ryan MJ. Flexibility in assessment of prey cues: frog-eating bats and frog calls. Proc R Soc B Biol Sci. 2005;272:841–847. doi: 10.1098/rspb.2004.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricelli GL, Blickley JL. Avian communication in urban noise: causes and consequences of vocal adjustment. Auk. 2006;123:639–649. [Google Scholar]
- Penna M, Solís R. Frog call intensities and sound propagation in the South American temperate forest region. Behav Ecol Sociobiol. 1998;42:371–381. [Google Scholar]
- Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol. 2003;18:228–233. [Google Scholar]
- Price TD. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J Exp Biol. 2006;209:2368–2376. doi: 10.1242/jeb.02183. [DOI] [PubMed] [Google Scholar]
- Pugesek BH, Tomer A, von Eye A. Structural equation modeling: applications in ecological and evolutionary biology. Cambridge (UK): Cambridge University Press; 2003. [Google Scholar]
- R Development Core Team. 2009. R: a language and environment for statistical computing. Vienna (Austria): R foundation for statistical computing [cited 2005 May 30]. Available from: http://www.R-project.org. [Google Scholar]
- Richards DG, Wiley RH. Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am Nat. 1980;115:381–399. [Google Scholar]
- Ryan MJ. Anuran communication. Washington (DC): Smithsonian Institution Press; 2001. [Google Scholar]
- Saunders J, Slotow R. The evolution of song structure in southern African birds: an assessment of the acoustic adaptation hypothesis. Ostrich. 2004;75:147–155. [Google Scholar]
- Scheiner SM. Plasticity as a selectable trait: reply to Via. Am Nat. 1993;142:371–373. [Google Scholar]
- Schwartz JJ. Call monitoring and interactive playback systems in the study of acoustic interactions among male anurans. In: Ryan MJ, editor. Anuran communication. Washington (DC): Smithsonian Institution Press; 2001. pp. 183–204. [Google Scholar]
- Shipley B. Cause and correlation in biology. A user's guide to path analysis, structural equations and causal inference. Cambridge (UK): Cambridge University Press; 2000. [Google Scholar]
- Shipley B. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge (UK): Cambridge University Press; 2002. [Google Scholar]
- Shy E, Morton ES. Adaptation of amplitude structure of songs to propagation in field habitat in song sparrows. Ethology. 1986;72:177–184. [Google Scholar]
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: WH Freeman; 1995. [Google Scholar]
- Via S. Adaptive phenotypic plasticity: target or by-product of selection in a variable environment? Am Nat. 1993;142:352–365. doi: 10.1086/285542. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Wiley RH. Associations of song properties with habitats for territorial oscine birds of eastern North America. Am Nat. 1991;138:973–993. [Google Scholar]
- Wiley RH, Richards DG. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav Ecol Sociobiol. 1978;3:69–94. [Google Scholar]
- Yao Y, Lin Y. Acoustic adaptation hypothesis in macro-and micro environments: an analysis of frog calls. J Acoust Soc Am. 2004;116:2639. [Google Scholar]
- Zimmerman B. A comparison of structural features of calls of open and forest habitat frog species in the central Amazon. Herpetologica. 1983;39:235–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.