Abstract
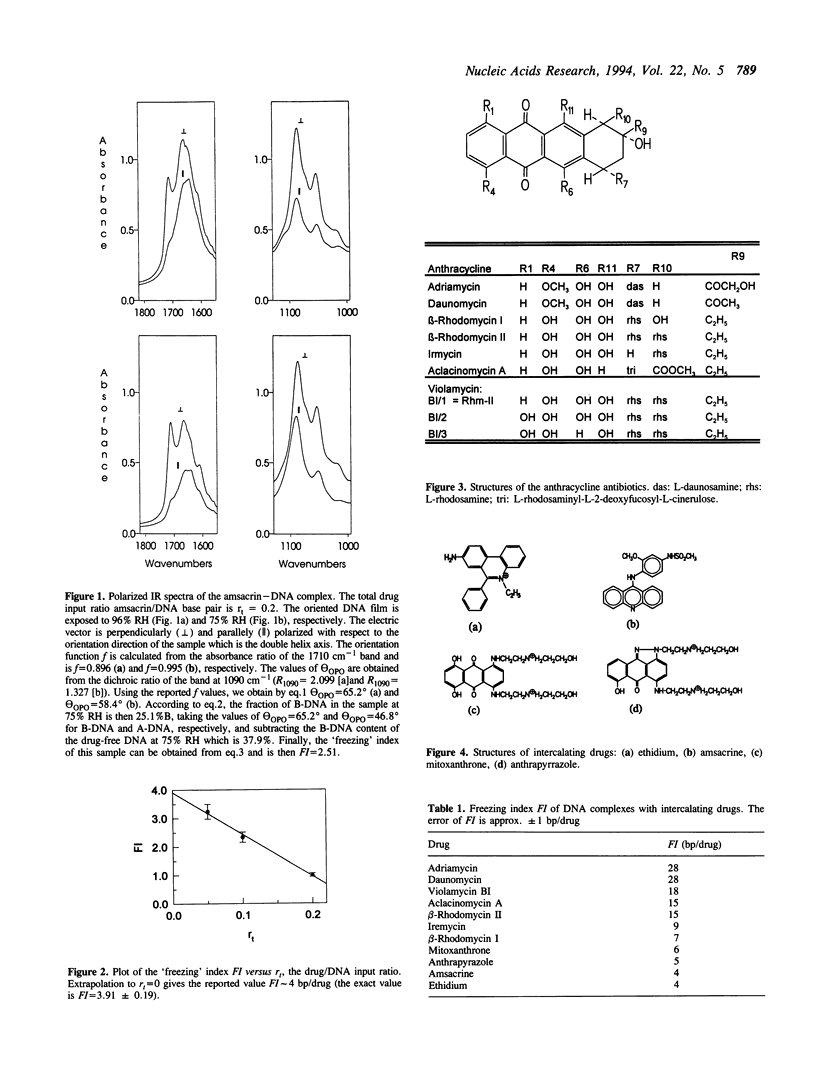
The B-A transition of films or fibers of NaDNA occurs at a relative humidity of 75-85%. The fraction of DNA that changed the conformation from B to A form can be determined quantitatively by infrared linear dichroism. DNA-binding drugs can 'freeze' a fraction of DNA in the B form. This fraction of DNA is in the B form and cannot be converted to A-DNA even at a reduced relative humidity of 54%. The 'freezing' potentiality of various drugs can be described by the 'freezing' index, FI, expressed in base pairs per added drug. Drugs with a high value of FI (more than eight base pairs per drug) were observed among both intercalating and groove-binding drugs. High values of FI imply restriction of the conformational flexibility of DNA significantly going beyond the binding site of the drug. This long-range effect of drugs on the conformational flexibility of DNA may be connected with the molecular mechanism of drug action. The freezing index FI is a new quantitative parameter of drug-DNA interaction that should be considered as a valuable tool for drug design.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandes R., Vold R. R., Kearns D. R., Rupprecht A. A 2H-NMR study of the A-DNA conformation in films of oriented Na-DNA: evidence of a disordered B-DNA contribution. Biopolymers. 1988 Jul;27(7):1159–1170. doi: 10.1002/bip.360270709. [DOI] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Chen K. X., Gresh N., Pullman B. A theoretical investigation on the sequence selective binding of daunomycin to double-stranded polynucleotides. J Biomol Struct Dyn. 1985 Dec;3(3):445–466. doi: 10.1080/07391102.1985.10508434. [DOI] [PubMed] [Google Scholar]
- Chuprina V. P., Lipanov A. A., Fedoroff OYu, Kim S. G., Kintanar A., Reid B. R. Sequence effects on local DNA topology. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9087–9091. doi: 10.1073/pnas.88.20.9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. J., Hamilton L. D. The A-B conformational change in the sodium salt of DNA. J Mol Biol. 1966 Apr;16(2):562–563. doi: 10.1016/s0022-2836(66)80193-6. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Kentebe E. Echinomycin binding to the sequence CG(AT)nCG alters the structure of the central AT region. Nucleic Acids Res. 1990 Apr 25;18(8):1957–1963. doi: 10.1093/nar/18.8.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche H., Rupprecht A. Modulation of the B-A transition of DNA by potential antitumor antibiotics. Influence of the base composition of DNA. J Biomol Struct Dyn. 1990 Apr;7(5):1135–1140. doi: 10.1080/07391102.1990.10508551. [DOI] [PubMed] [Google Scholar]
- Fritzsche H., Rupprecht A., Richter M. Infrared linear dichroism of oriented DNA-ligand complexes prepared with the wet-spinning method. Nucleic Acids Res. 1984 Dec 11;12(23):9165–9177. doi: 10.1093/nar/12.23.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. G., Sriram M., Denny W. A., Wang A. H. Minor groove binding of SN6999 to an alkylated DNA: molecular structure of d(CGC[e6G]AATTCGCG)-SN6999 complex. Biochemistry. 1993 Sep 21;32(37):9639–9648. doi: 10.1021/bi00088a016. [DOI] [PubMed] [Google Scholar]
- Grimm H., Rupprecht A. Hydration structure in natural DNA observed by thermal neutron scattering. Eur Biophys J. 1989;17(4):173–186. doi: 10.1007/BF00284723. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Krylov DYu A-DNA in solution as studied by diverse approaches. Methods Enzymol. 1992;211:111–127. doi: 10.1016/0076-6879(92)11008-7. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Schyolkina A. K. Cooperative transitions in DNA with no separation of strands. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):243–250. doi: 10.1101/sqb.1983.047.01.029. [DOI] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. C., Sokolov N. V., He C. M., Setlow P. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):77–81. doi: 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden B., Kubista M., Kurucsev T. Linear dichroism spectroscopy of nucleic acids. Q Rev Biophys. 1992 Feb;25(1):51–170. doi: 10.1017/s0033583500004728. [DOI] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Pilet J., Brahms J. Dependence of B-A conformational change in DNA on base composition. Nat New Biol. 1972 Mar 29;236(65):99–100. doi: 10.1038/newbio236099a0. [DOI] [PubMed] [Google Scholar]
- Pohle W., Zhurkin V. B., Fritzsche H. The DNA phosphate orientation. Infrared data and energetically favorable structures. Biopolymers. 1984 Nov;23(11 Pt 2):2603–2622. doi: 10.1002/bip.360231131. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht A. A wet spinning apparatus and auxiliary equipment suitable for preparing samples of oriented DNA. Biotechnol Bioeng. 1970 Jan;12(1):93–121. doi: 10.1002/bit.260120109. [DOI] [PubMed] [Google Scholar]
- Rupprecht A. Preparation by wet spinning of oriented DNA films for polarized infrared study. Biochim Biophys Acta. 1970 Jan 21;199(1):277–280. doi: 10.1016/0005-2787(70)90716-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]


