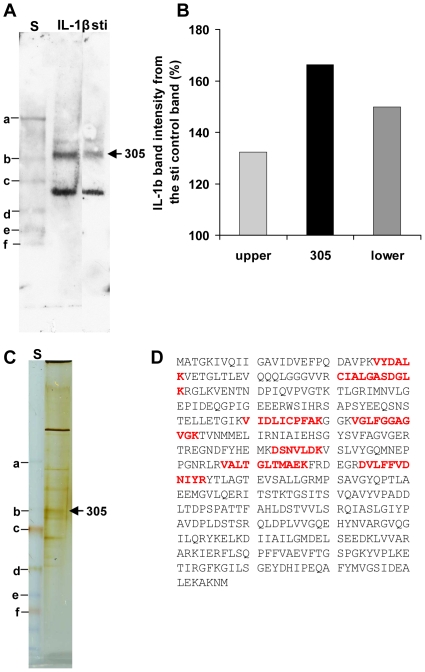
Figure 5. Binding of IL-1β to intracellular protein fraction of A. actinomycetemcomitans.
The samples were run in native-PAGE, and the proteins were transferred to nitrocellulose membrane. The reactive bands were then detected using biotinylated IL-1β and, HRP-linked streptavidin. Control detection included biotinylated soybean trypsin inhibitor (sti). Reactive protein band 305 (named according to its travelling distance in these gels), which was more pronounced in IL-1β incubated detection than in the control detection, was isolated from identical gel after silver staining. The isolated protein band 305 was digested with trypsin before peptide separation and peptide identification with LC-MS/MS. Panel A shows the blots treated with either IL-1β or sti. Panel B shows the intensities of the three reactive bands in IL-1β-treated membranes compared to sti-treated membranes. Panel C presents the silver stained native-PAGE gel. Panel D shows the seven peptides that were identified from protein band 305 (denoted by the sequences given in bold type). According to the peptide sequence data, the protein was identified as ATP synthase subunit β (Aggregatibacter actinomycetemcomitans D11S-1) [42]. The Mascot program combined with Sprot-Trembl (uniprot) protein sequence database was used to the protein identification. The gels in Panels A and B contained PageRuler™ Plus Prestained Protein Ladder (denoted by “S”), and some of the proteins in the ladder are denoted (a–f).