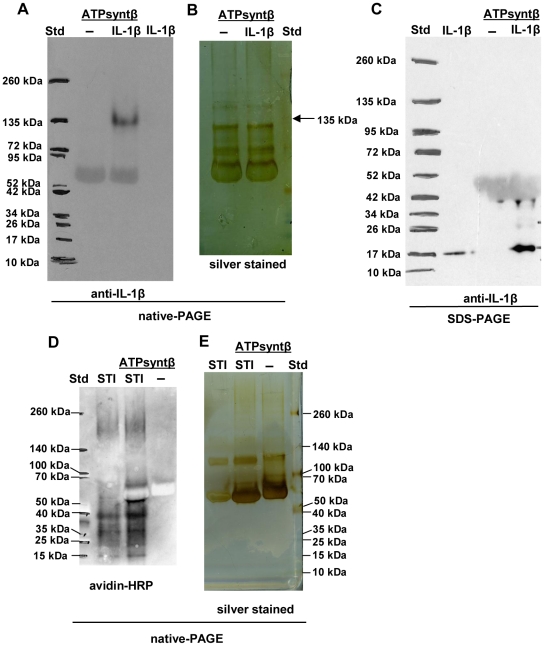
Figure 6. IL-1β binding capacity of recombinant ATP synthase subunit β of A. actinomycetemcomitans.
Recombinant ATP synthase subunit β (88 µM) was incubated with or without IL-1β (0.29 µM) for 1 h, after which the samples were run in native-PAGE and immunoblotted with anti-IL-1β (Panel A), or silver stained (Panel B). Three prominent forms could be observed from recombinant ATP synthase subunit β (Panel B). IL-1β bound to the trimeric form of recombinant ATP synthase subunit β. IL-1β was not detectable from immunoblotted native-PAGE without pre-incubation with ATP synthase subunit β (Panel A). However, IL-1β was released from the trimeric form of ATP synthase subunit β under denaturing conditions of SDS-PAGE (Panel C). The sizes of ATP synthase subunit β and IL-1β were 51 kDa and 17 kDa, respectively. The binding of the biotinylated control protein soy bean trypsin inhibitor (STI) to ATP synthase subunit β was was estimated similarly (Panel D and E) by using streptavidin-HRP in detection, except the SDS-page was not run. The control protein bound only to the ATP synthase subunit β monomer.