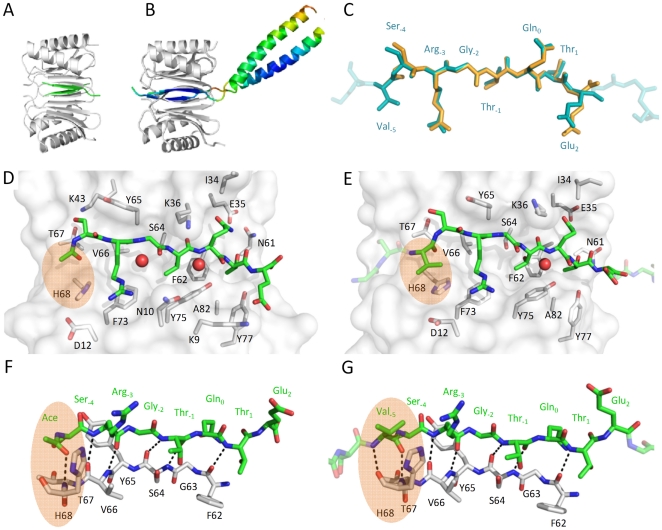
Figure 4. Crystal structure of Ac-SRGTQTE – DYNLL2 (A, C, D and F) and Leu-zipper dimerized VSRGTQTE – DYNLL2 complex (B, C, E and G).
Peptides bound to DYNLL adopt similar conformation, see alignment in C. As shown in B the Leu-zipper dimerized VSRGTQTE peptides lay into two parallel binding grooves on DYNLL2 forming a dimer-dimer complex. Colors from red to blue correspond to high and low B-factors, respectively (from 131 to 48). The GGSG linker between the binding motif and the Leu-zipper appears to be flexible. Side chain interactions between the peptides and the binding grove of DYNLL2 are shown in D and E. Side chains of DYNLL2 having at least one atom within 4 Å distance from the peptide are shown as sticks. F and G are rotated versions (by approximately 90° along the axes of the binding motifs) of D and E, respectively, and show that the peptides bind to DYNLL2 in a β-strand conformation. Interestingly, the interaction is partially mediated by H-bridges of several buried structural waters represented as red spheres in D and E. Comparing F and G shows that the backbone of Val-5 forms one additional hydrogen bond, while E and G illustrate that its side chain interacts with DYNLL2 His68. Details of this interaction are highlighted by orange areas.