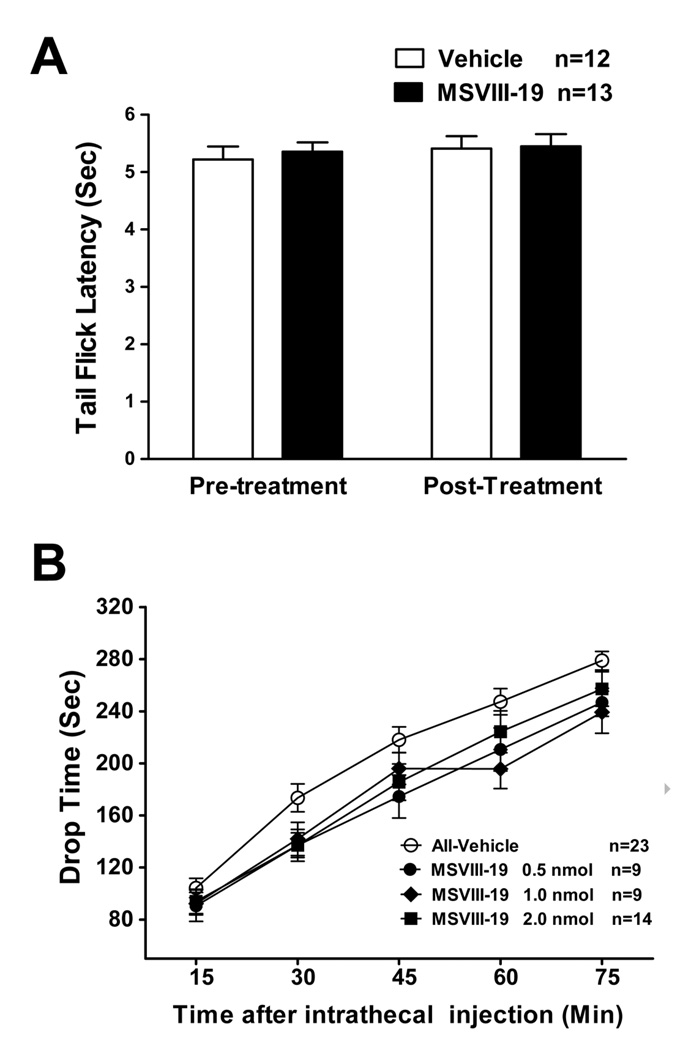
Figure 7. MSVIII-19 has no effect in the tail-flick assay of thermal pain and has no effect on locomotor behavior in the accelerating rotarod.
A) Mice were first tested for baseline tailflick latency. (Pre-inj). They then received an intrathecal injection of MSVIII-19 (0.5nmol), and tailflick latency was then measured again (Post-inj). There was no difference between MSVIII-19 (0.5 nmol) and vehicle-injected groups. B) The effects of intrathecial MSVIII-19 (0.5–2.0nmol) were compared to vehicle-treated mice in locomotor performance on the accelerating rotarod. Mice were pretreated with an intrathecal injection of vehicle (n=23) or MSVIII-19 (0.5 nmol, n=9; 1.0 nmol, n=9; 2.0 nmol, n=14). There were no significant effects of MSVIII-19 as compared to vehicle.