Table 1.
Summary of Clinical, Pathologic, and Treatment Outcome Data for the 11 Study Participants
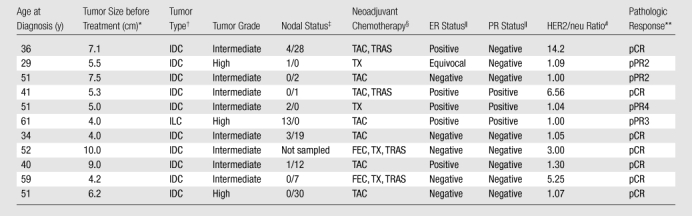
Maximum dimension at pretreatment magnetic resonance (MR) imaging.
IDC = infiltrating ductal carcinoma, ILC = infiltrating lobular carcinoma.
Number of axillary nodes positive for metastatic tumor/number of axillary nodes negative for metastatic tumor.
FEC = fluorouracil, epirubicin, and cyclophosphamide; TAC = docetaxel, doxorubicin, and cyclophosphamide; TRAS = trastuzumab; TX = paclitaxel or docetaxel.
According to the immunohistochemical (IHC) semiquantitative scoring system used, > 15% IHC staining = positive, 0% IHC staining = negative, and 1%–14% IHC staining = equivocal. ER = estrogen receptor, PR = progesterone receptor.
HER2/neu = human epidermal growth factor receptor 2. Gene analysis was performed with fluorescence in situ hybridization, with results compared against a normal control. A ratio < 2.0 = normal expression, a ratio between 2.0 and 4.0 = equivocal expression, and a ratio > 4.0 = gene overexpression.
pCR = complete pathologic response with either no residual carcinoma or no residual invasive tumor but ductal carcinoma in situ present; pPR = partial pathologic response, subclassified as pPR1 (near total treatment effect [<10% of tumor remaining]), pPR2 (evidence of response to therapy but with 10%–50% of tumor remaining), pPR3 (evidence of response to therapy but with between 50% and 90% of tumor remaining), and pPR4 (minimal evidence of response to therapy with > 90% of tumor remaining).
