Table 4.
A regioselective 1,3-hydrogen shift.a
| entry | allenamides | conditions (time) | amido-alkenes | yield [%]b,c | ||
| 1 | 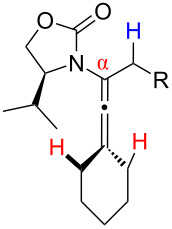 |
29a: R = Ph | 115 °C (16 h) | 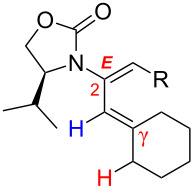 |
33a | 71 |
| 2 | 29b: R = H | —d | 33b | 90 | ||
| 3 | 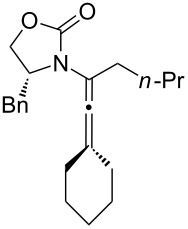 |
30 | 115 °C (16 h) | 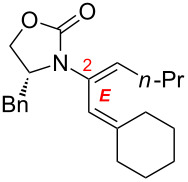 |
34 | 79 |
| 4 | 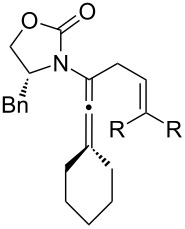 |
31a: R = H | CSA (10 min) |  |
35a | 68 |
| 5 | 31b: R = Me | CSA (10 min) | 35b | 80 | ||
| 6 | 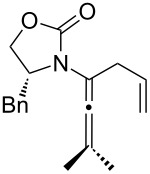 |
32 | CSA (10 min) | 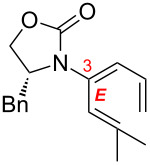 |
36 | 84 |
aUnless otherwise noted, CH3CN was the solvent for thermal conditions and CH2Cl2 was the solvent when using 10 mol % of CSA at rt. For all reactions, concn = 0.10 M. bAll were isolated yields. cAll amido-di- and trienes were exclusively E-selective [≥95:5]. dSee text for this isomerization.
