FIG. 6.
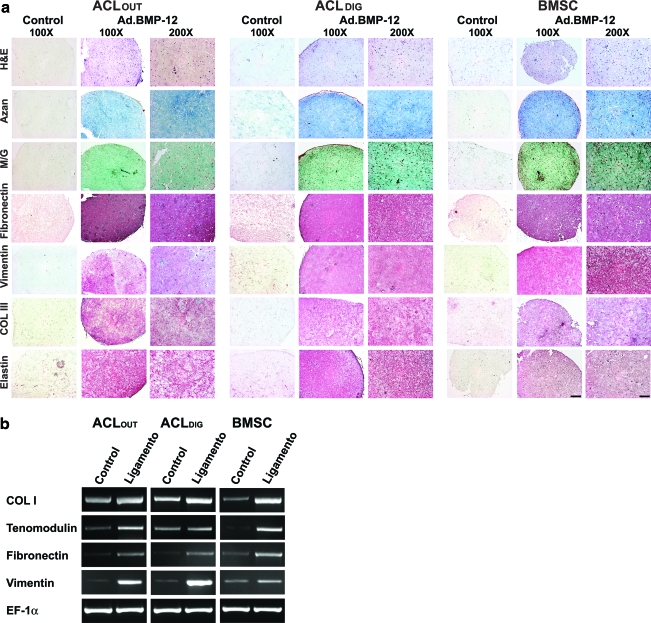
Ligamentogenic differentiation of ACLOUT versus ACLDIG and BMSC cultures. As self-renewal is an important stem cell criterion, we employed ACLOUT, ACLDIG, and BMSC populations in an in vitro assay of ligamentogenesis using bone morphogenetic protein 12 (BMP12) encoding adenovirus vectors and three-dimensional culture in collagen hydrogels for differentiation induction compared to controls, which were maintained after marker gene transduction (green fluorescent protein, GFP) in a similar fashion. (a) At 21 days, hematoxylin and eosin (H&E) stainings revealed homogenous cell distribution of fibroblasts for all cell types within the hydrogel constructs (first row), and a strong accumulation of a collagenous matrix after modifications with BMP12 compared to GFP-modified controls where less matrix formation was seen, as evidenced by matrix staining with Azan (second row) and Masson/Goldner (M/G; third row). Immunohistochemical analyses for the ligament matrix proteins fibronectin (fourth row), vimentin (fifth row), collagen type III (Col III; sixth row), and elastin (seventh row) revealed more intense stainings in the Ad.BMP12 transduced hydrogel constructs of all cell types compared to corresponding controls, where no red staining of the matrix was detectable. Differences between cell types (ACLOUT vs. ACLDIG vs. BMSC) could not be detected. Scale bars: 100×bar=200 μm; 200×bar=100 μm. (b) RT-PCR analysis for expression of ligament-specific genes revealed that ACLOUT, as well as ACLDIG and BMSCs, increased expression of the factors COL I, tenomodulin, fibronectin, and vitronectin compared to the respective GFP controls, where only weak expression levels could be detected. Expression of EF-1α was included as a constitutive control for RNA loading. Color images available online at www.liebertonline.com/tea