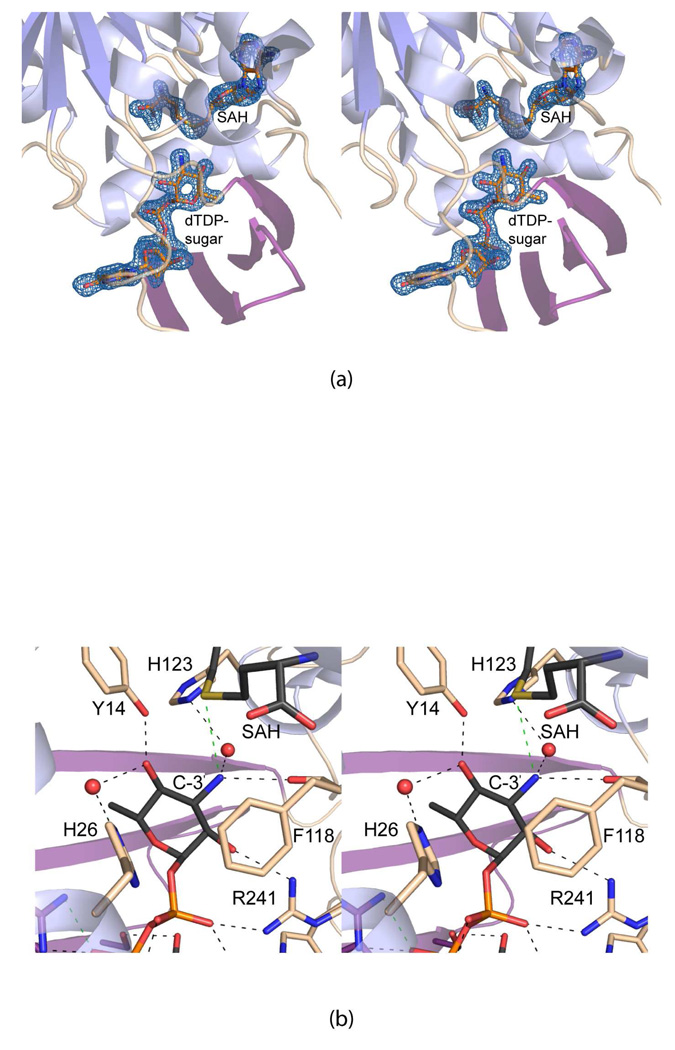
Figure 4.
Active site of the TylM1/SAH/dTDP-Quip3N complex. The electron densities corresponding to the SAH and dTDP-Quip3N ligands are shown in (a). The map was contoured at 2.5σ and calculated with coefficients of the form (Fo − Fc), where Fo was the native structure factor amplitude and Fc was the calculated structure factor amplitude. Potential hydrogen bonds between the protein and the hexose moiety of dTDP-Quip3N are indicated by the dashed lines in (b). Water molecules are represented as red spheres. The green dashed line indicates a distance of 3.6 Å between the amino group of the sugar and the sulfur of SAH.