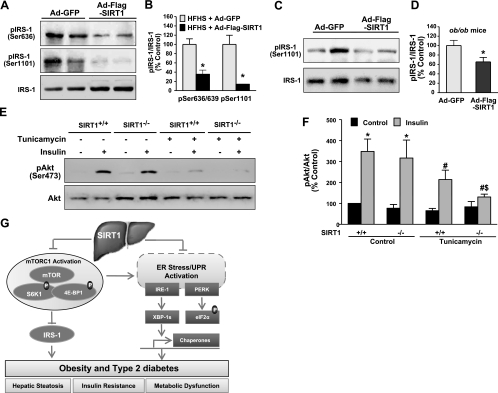
Figure 8.
Modulated SIRT1 activity regulates insulin signaling in the liver of diet- and obesity-induced insulin-resistant mice and in vitro. A, B) Insulin receptor signaling is enhanced by hepatocellular SIRT1 overexpression in diet-induced insulin-resistant LDLR−/−mice. Phosphorylation of IRS-1 at Ser636/639 and Ser1101, two major specific phosphorylation sites of S6K1, was decreased in the liver of insulin-resistant mice with Ad-Flag-SIRT1 injection. A) Representative immunoblots of phosphorylated IRS-1 and total proteins from 2 mouse livers/group. B) Relative phosphorylation levels were normalized to those of endogenous proteins. C, D) IRS-1 phosphorylation at Ser636/639 in the liver of ob/ob mice is prevented by overexpressing SIRT1. C) Representative immunoblots of phosphorylated IRS-1 and total proteins from 2 mouse livers/group. D) Relative phosphorylation levels were normalized to those of endogenous proteins. Data are presented as means ± se (n=5–6). *P < 0.05 vs. Ad-GFP group. E, F) SIRT1 deficiency impairs insulin-stimulated phosphorylation of Akt under the conditions of ER stress. E) SIRT1+/+ and SIRT1−/− MEFs were cultured quiescently in serum-free medium for 3 h, pretreated with tunicamycin (2 μg/ml) for 6 h, and then stimulated with insulin (100 nM) for 10 min. Cell lysates were subjected to immunoblotting for phosphorylation of Akt. F) Data are presented as means ± se (n=4). *P < 0.05 vs. untreated control group; #P < 0.05 vs. insulin stimulation group; $P < 0.05 vs. tunicamycin treatment and insulin stimulation group. G) Proposed model for SIRT1 activation to attenuate obesity-induced ER stress, hepatic steatosis, and insulin resistance in large part through inhibition of mTORC1. In diet- and obesity-induced T2DM, chronic energy and nutrient excess activate nutrient-sensing pathway of mTORC1 and S6K1, which leads to the feedback inhibition of insulin receptor signaling through serine phosphorylation of IRS-1. Simultaneously, obesity-induced hepatic ER stress activates the UPR that includes PERK-mediated phosphorylation of eIF2α, IRE-1-mediated splicing of XBP-1, and transcription of UPR target genes, GRP78 and CHOP. Hyperactivation of mTORC1 also results in uncontrolled protein synthesis that triggers a vicious cycle to accelerate the development of more severe ER stress in the liver, ultimately culminating in systemic insulin resistance and metabolic dysfunction. Conversely, SIRT1 activation in the liver may counteract the mTORC1/S6K1 activation in diet- and obesity-induced insulin resistance and, in turn, prevent the vicious cycle by restoring hepatocellular ER homeostasis and by blocking S6K1-mediated feedback inhibition of IRS-1—all of which modulates insulin action on hepatic glucose metabolism and enhances systemic insulin sensitivity. The identification of coordinated regulation between nutrient sensing pathways of SIRT1 and mTORC1 provides novel mechanistic insights into potential targets for treatment of ER stress and hepatic steatosis in obesity and T2DM.