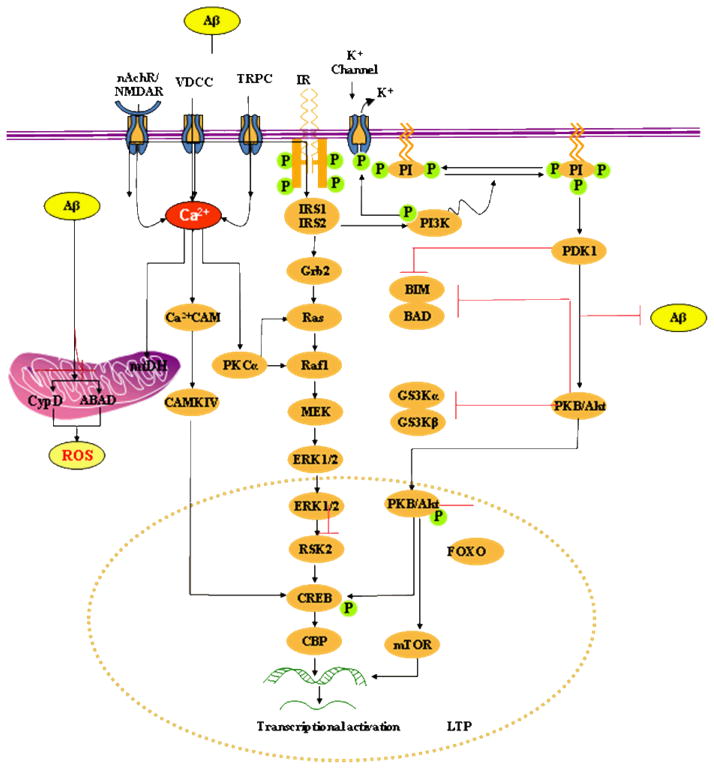
Fig. 1. Model of picomolar Aβ-induced insulin-PI3K-Akt-ERK signalling plus mitochondrial targets of intracellular Aβ.
Extracellular Aβ at picomolar concentration binds to the insulin receptor (IR) and activates PKB/Akt via PDK-1. PKB/Akt translocates into the nucleus and phosphorylates CREB. Activation of the lipid kinase PI3K is critical for the activation of PKB by PDK. PDK1 phosphorylates the activation loop of a number of protein serine/threonine kinases of the AGC kinase superfamily, including protein kinase B (PKB α; also called Akt1). Akt may also maintain the integrity of the mitochondria by a unknown mechanism or by a specific mechanism of Bad phosphorylation. Akt can also inhibit apoptosis by phosphorylation and inactivation of caspase-9. ERK1/2 are activated by upstream MAPKK, such as MEK1/2, and MAPKKK, such as c-Raf. MEK1/2 induce ERK1/2 activation via dual phosphorylation on threonine 202 and tyrosine 204 residues. Phosphorylation of ERK leads to the activation of a number of transcription factors, important in controlling differentiation, neuronal survival, learning and memory plasticity. For example, ERK activates pro-survival transcription factor CREB, by activating both p90RSK and MSK1/2.
Picomolar extracellular Aβ also binds nAChR, glutamate receptors (NMDAR) and Ca2+ ion channels (e.g. VDCCs, TRPC) and causes Ca2+ influx at controlled rates into the cytoplasm and mitochondria. Increased cytosolic calcium concentrations initiate the activation of several kinase-dependent signalling cascades including activation of PKC leading to CREB activation and phosphorylation at Ser133, a process critical for protein synthesis-dependent synaptic plasticity and LTP. PKC-α also activates ERK by interacting with Ras or Raf-1.
Mitochondria are critical targets of intracellular Aβ. Aβ interacts with CypD, a protein component of the membrane permeability transition pore (MPTP). The interaction of CypD with Aβ causes functional modification of this protein leading to MPTP opening. Aβ also binds with another mitochondrial protein, ABAD to distort the enzyme’s structure, rendering it inactive. This causes an increase in reactive oxygen species and oxidative stress leading to initiation of apoptosis.