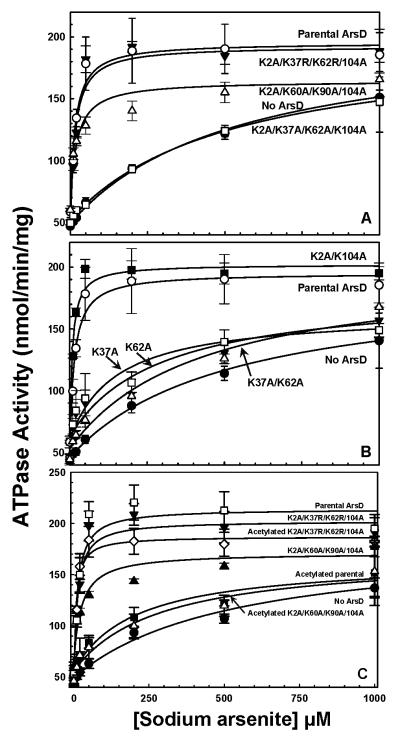
Fig. 3. Activation of ArsA ATPase activity by ArsD lysine mutants and abolition of ArsD metallochaperone activity by acetylation.
Activated ArsA ATPase activity was determined at the indicated concentrations of sodium arsenite in the presence or absence of ArsD derivatives, as described under Materials and Methods. The concentration of ArsA was 0.3 μM, and ArsD was 6 μM. A. The concentration of As(III) required for half maximal activation is given in parentheses. (●), no ArsD (K1/2 = 540 μM); (○), ArsD (K1/2 = 20 μM); (Δ), K2A/K60A/K90A/K104A (K1/2 = 20 μM); (▼), K2A/K37R/K62R/K104A (K1/2 = 20 μM); (□), K2A/K37A/K62A/K104A (K1/2 = 520 μM). B. (●), no ArsD (K1/2 = 510 μM); (○), ArsD (K1/2 = 20 μM); (■),K2A/K104A (K1/2 = 10 μM); (□), K37A (K1/2 = 150 μM); (▼), K62A (K1/2 = 290 μM); (Δ), K37A/K62A (K1/2 = 430 μM). C. (●), no ArsD (K1/2 = 520 μM); (□), ArsD (K1/2 = 10 μM); (■), acetylated ArsD (K1/2 = 200 μM); (▲), K2A/K60A/K90A/K104A (K1/2 = 30 μM); (Δ), acetylated K2A/K60A/K90A/K104A (K1/2 = 280 μM); (▼), K2A/K37R/K62R/K104A (K1/2 = 10 μM); (◇), acetylated K2A/K37R/K62R/K104A (K1/2 = 10 μM). The data were fitted using SigmaPlot 9.0, with error bars representing the standard deviation from three assays.