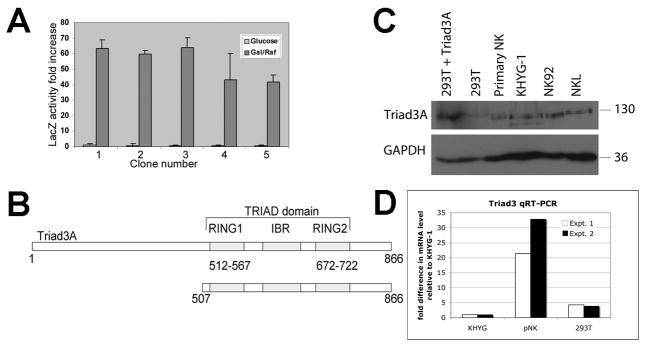
FIGURE 1. Identification of Triad3A as a 2DL4 binding partner by yeast two hybrid screening.
The 2DL4 cytoplasmic domain (aa 858–1032) was used to identify interacting proteins in a yeast two-hybrid screen. A, Five positive clones identified in a growth assay, which all encoded the C-terminus of Triad3A, also tested positive for β-galactosidase reporter activity. Results are mean fold increase LacZ activity ±S.D. comparing inducing conditions (galactose/raffinose; Gal/Raf) to uninduced conditions (glucose; activity = 1) from 3 assays. B, The 2DL4-interacting portion of Triad3A protein identified in all 5 clones (aa 507–866; bottom) includes the Triad domain [two RING domains and IBR domain] from the full protein (top). C, Immunoblotting for expression of Triad3 and GAPDH in total lysates of primary NK cells, KHYG-1 NK cell line, NK-92 NK cell line, NKL NK cell line, 293T cells, and 293T cells transfected with Triad3A cDNA. Results are representative of at least 3 experiments. D, qRT-PCR analysis of Triad3 mRNA expression in KHYG-1, primary NK, and 293T cells. Results from two experiments were normalized to GAPDH mRNA levels in each cell type and expressed as fold difference as compared to the amounts detected in KHYG-1 cells, which were set at a relative value of 1.