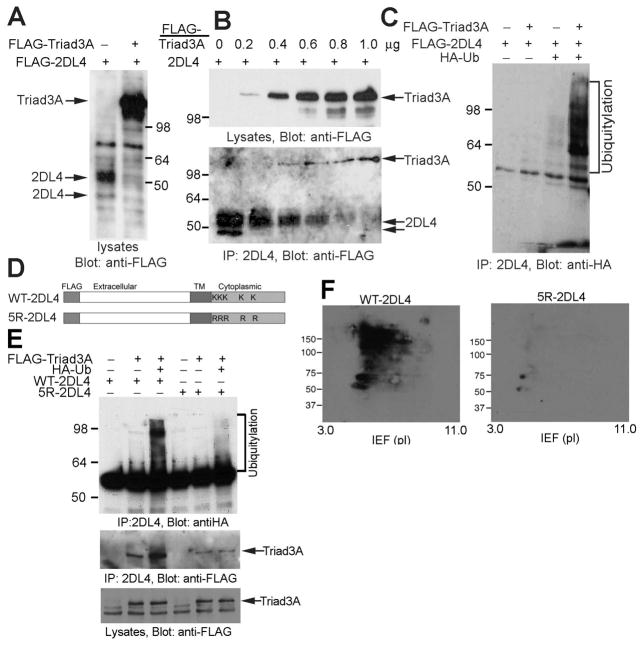
FIGURE 2. Triad3A promotes polyubiquitylation and degradation of 2DL4 in 293T cells.
A, Co-expression of Triad3A reduces 2DL4 expression in 293T cells. 293T cells were transfected with FLAG-2DL4 ± FLAG-Triad3A, lysed 48 h later, separated by SDS-PAGE, and immunoblotted with anti-FLAG mAb. B, Triad3A associates with and induces degradation of 2DL4 protein. 293T cells were co-transfected with indicated amount of FLAG-Triad3A- and 1 μg of FLAG-2DL4-encoding plasmids, lysed 48 h later (lysates in top panel), and 2DL4 immunoprecipitates (IP in bottom panel) were immunoblotted with anti-FLAG mAb. C, Triad3A induces ubiquitylation of 2DL4. 293T cells were transfected with indicated cDNAs, lysed 48 h later, immunoprecipitated with anti-2DL4 mAb, and analyzed for Ub by anti-HA immunoblotting. D. Structure of WT-2DL4 and the 5R-2DL4 constructs (TM = transmembrane, K = Lys, R = Arg). E. Direct polyubiquitylation of lysines in the cytoplasmic domain of 2DL4 by Triad3A. 293T cells were transfected with indicated combinations of cDNAs, lysed 48 h later, immunoprecipitated with anti-2DL4 mAb, and immunoblotted for Ub with anti-HA Ab. Immunoprecipitates and lysates were also probed with anti-FLAG mAb to detect Triad3A (bottom). F. Analysis of lysine-dependent ubiquitylation of 2DL4 by 2D gel electrophoresis. 293T cells were transfected with FLAG-Triad3A, HA-Ub, and FLAG-WT-2DL4 (left) or FLAG-5R-2DL4 (right), lysed 48 h later, immunoprecipitated with anti-2DL4 mAb, separated by 2D gel electrophoresis (IEF/SDS-PAGE), and immunoblotted for Ub with anti-HA Ab.