Table 3.
Attempted elimination conditions.
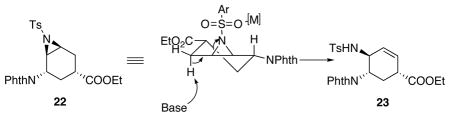 | ||
|---|---|---|
| Entry | Conditions | Notes |
| 1 | 2 equiv. LDA, THF, −78 °C | Decomposition to baseline by TLC |
| 2 | 1 equiv. KOtBu, THF, 0 –40 °C | No desired product |
| 3 | 5 equiv. KOtBu, THF, rt | Messy, t-BuO− attacked ester |
| 4 | 1 eq LIDAKOR[a], hexanes, 0 °C-rt | NR (substrate not dissolving) |
| 5 | 1 eq LIDAKOR, THF, −78 °C | Decomposition to baseline by TLC |
| 6 | 1.5 eq DBU, toluene, reflux | NR |
| 7 | 50 mol% In(OTf)3, 2,6-di-tert-butylpyridine, THF, 50 °C | NR |
| 8 | 20 mol% In(OTf)3, proton-sponge, THF, reflux | No desired reaction. In(OTf)3 reacted with proton-sponge to form insoluble solids in the reaction. |
| 9 | Ho(OTf)3, 2,6-di-tert-butylpyridine, chlorobenzen, 100 °C | NR, recovered starting material |
| 10 | Al(OTf)3, 2,6-di-tert-butylpyridine, DCM, 0 °C-rt | 100% NMR yield[c] of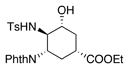 24 |
| 11 | In(OTf)3, 2,6-di-tert-butylpyridine, chlorobenzene, 100 °C | NMR yields: 23% 23, 30% 24,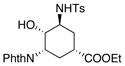 25 30% |
| 12 | In(OTf)3, 2,6-di-tert-butylpyridine, chlorobenzene, 3Å MS, 100 °C | NR, recovered starting material |
LIDAKOR=LDA + tBuOK.
NR = no reaction
NMR yields were determined by using 1,3,5-trimethoxybenzene as an internal standard.
