Table 6.
Selected conditions for the aziridination of diene 38.
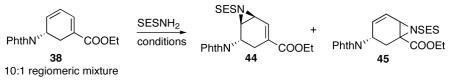 | |||
|---|---|---|---|
| Entry | Catalyst | Conditions | Result[b] |
| 1 | 10 mol% Cu(CH3CN)4PF6 | PhI=O; 3 A MS; CH3CN; rt | 84%; 44 : 45 = 1.7 : 1 |
| 2 | 10 mol% Cu(IPr)Cl[c] + AgBF4 | PhI=O; 3 A MS; CH3CN; 0 °C | (80%); 44 : 45 = 1.6 : 1 |
| 3 | 10 mol% Ag2(tBu3tPy)2(NO3)2[d] | PhI=O; 3 A MS; CH3CN; 0 °C-rt | (< 48%);[e] 44 only |
| 4 | 10 mol% Au(tBu3tPy)OTf[d] | PhI(OAc)2; 4 A MS; CH3CN; 80-50 °C | (< 53%);[e] 44 only |
| 5 | 2 mol% Rh2(CF3CONH)4 | PhI(OAc)2; MgO; C6H5Cl; 0 °C-rt | (18%); 44 only |
| 6 | 5 mol% Rh2(OCOCPh3)4 | PhI(OAc)2; MgO; C6H5Cl; 0 °C-rt | (35%); 44 only |
| 7 | 5 mol% Rh2(OCOCMe3)4 | PhI(OAc)2; MgO; C6H5Cl; 0 °C-rt | (56%); 44 only |
| 8 | 5 mol% Rh2(esp)2 | PhI(OAc)2; MgO; C6H5Cl; 0 °C-rt | (71%); 44 only |
| 9 | 2 mol% Rh2(esp)2 | PhI(OAc)2; MgO; C6H5Cl; 0 °C-rt | (72%); 44 only |
| 10 | 2 mol% Rh2(esp)2 | PhI(OPiv)2; MgO; C6H5Cl; 0 °C-rt | 86% (91%); 44 only |
| 11 | 2 mol% Rh2(esp)2 | PhI(OPiv)2; MgO; isopropyl acetate; 0 °C-rt | (91%);[f] 44 only |
The stereochemistry of 45 is undetermined. Compound 44 and 45 are inseparable on column.
Yields in parentheses were determined by 1H NMR with 1,3,5-trimethoxybezene as an internal standard.
IPr = 1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene.
tBu3tPy = 4,4′,4″-tri-tert-butyl-2,2′;6′,2″-terpyridine.
Conversion was accompanied by decomposition, resulting in irreproducible results.
Dry isopropyl acetate (IPAC), a non-halogenated solvent, gave the same result as dry chlorobenzene.
