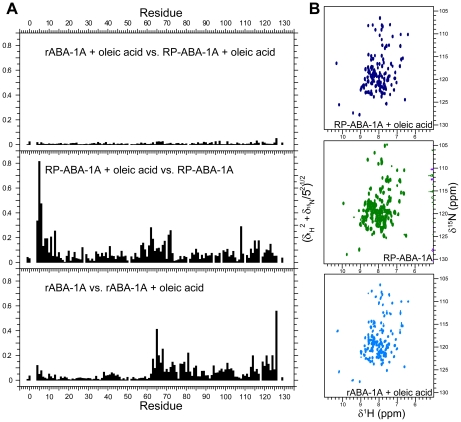
Figure 5. NMR evidence for ligand binding by rABA-1A.
A) Backbone amide chemical shift perturbation (√[|δ1H|2+(0.2|δ15N|)2]) reporting on changes in structure between different forms of ABA-1A: recombinant ABA-1A purified from E. coli (rABA-1A); rABA-1A saturated with oleic acid; rABA-1A stripped of resident ligand(s) by reverse phase chromatography (RP-ABA-1A); and RP-ABA-1A saturated with oleic acid. Saturation of either rABA-1A or RP-ABA-1A with oleic acid gives rise to essentially identical backbone amide chemical shifts and therefore structures. Compared with the oleic acid bound form, rABA-1A has different shifts in its C-terminal half indicating that cavity II is not occupied, or occupied by a different ligand in this form. In contrast RP-ABA-1A displays shifts in both N-and C-termini implying that both cavites are empty in this form. B) 15N HSQC spectra of RP-ABA-1A, rABA-1A and RP-ABA-1A saturated with oleic acid.