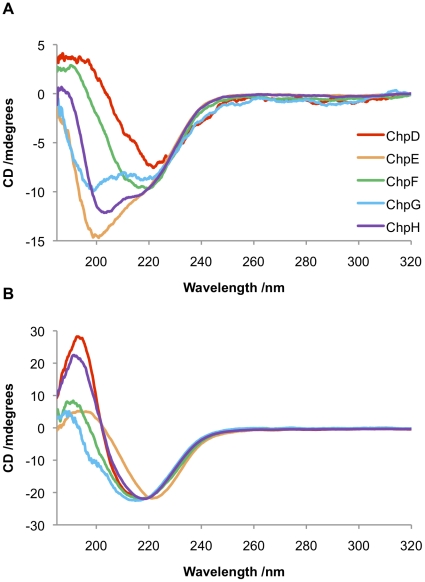
Figure 2. Characterisation of the secondary structures of chaplin peptides by CD spectroscopy.
(A) Peptides in water without agitation. ChpD (red) and ChpF (green) are comprised largely of β-sheet; ChpE (orange) is predominantly random coil; while the spectra of ChpG (blue) and ChpH (purple) are indicative of a mixed secondary structure comprising elements of both β-sheet and random coil. (B) Upon vortexing all of the chaplins adopt a β-sheet rich secondary structure.