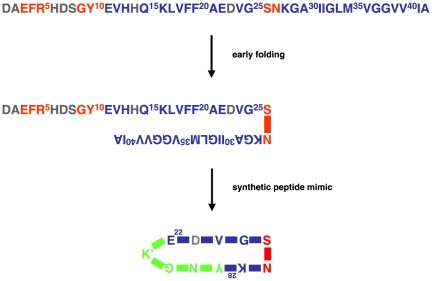
Figure 1. Early folding of human amyloid β (Aβ), around S26 and N27.
The figure is a simplification of the model designed by Olofsson et al. [28]. Residues in red are solvent accessible; residues in blue (and to a lesser extent in gray) are shielded from the solvent. The peptide cyclo[Aβ(22–28)-YNGK′] is a mimic for misfolded Aβ. YNGK′ is a turn-stabilizing sequence and K′ is a side-chain-modified lysyl residue for selective conjugation to a protein carrier. Other YNGK′-containing cyclic peptides prepared (23–28, 24–29, 25–30, 21–27, 22–28, 23–29, 24–30, and 25–31) did not mimic misfolded Aβ.