Abstract
The serotonergic neurotoxin, 3,4-methylenedioxymethamphetamine (MDMA/Ecstasy), is a highly popular recreational drug. Human recreational MDMA users have neurocognitive and neuropsychiatric impairments, and human neuroimaging data are consistent with animal reports of serotonin neurotoxicity. However, functional neuroimaging studies have not found consistent effects of MDMA on brain neurophysiology in human users. Several lines of evidence suggest that studying MDMA effects in visual system might reveal the general cortical and subcortical neurophysiological consequences of MDMA use. We used 3 T functional magnetic resonance imaging during visual stimulation to compare visual system lateral geniculate nucleus (LGN) and Brodmann Area (BA) 17 and BA 18 activation in 20 long abstinent (479.95±580.65 days) MDMA users and 20 non-MDMA user controls. Lifetime quantity of MDMA use was strongly positively correlated with blood oxygenation level-dependent (BOLD) signal intensity in bilateral LGN (rs=0.59; p=0.007), BA 17 (rs=0.50; p=0.027), and BA 18 (rs=0.48; p=0.031), and with the spatial extent of activation in BA 17 (rs=0.059; p=0.007) and BA 18 (rs=0.55; p=0.013). There were no between-group differences in brain activation in any region, but the heaviest MDMA users showed a significantly greater spatial extent of activation than controls in BA 17 (p=0.031) and BA 18 (p=0.049). These results suggest that human recreational MDMA use may be associated with a long-lasting increase in cortical excitability, possibly through loss of serotonin input to cortical and subcortical regions. When considered in the context of previous results, cortical hyper-excitability may be a biomarker for MDMA-induced serotonin neurotoxicity.
Keywords: neurotoxicity, amphetamine, cortical excitability, serotonin, drug abuse
INTRODUCTION
The substituted amphetamine, 3,4-methylenedioxymethamphetamine (MDMA/Ecstasy), is widely used as a recreational drug owing to its acute stimulant, hallucinogenic, and pro-social effects that are produced by MDMA's acute actions on brain serotonin (5-HT), dopamine, and norepinephrine (Bankson and Cunningham, 2001). Despite evidence that MDMA causes long-lasting serotonergic neurotoxicity, MDMA use remains high in the United States and European Union (NSDUH, 2009; EMCDDA, 2010). Because of MDMA's effects on neurotransmitter physiology (Yamamoto et al, 2010) and case reports suggesting that it may facilitate insight and engagement (Liester et al, 1992), MDMA is in clinical research trials as an adjunct to psychotherapy for anxiety disorders (NIH, 2008a, 2008b), opening the possibility that MDMA may become an approved pharmacotherapeutic agent.
In this context of widespread recreational exposure and potential therapeutic administration, much remains unknown with regard to the long-lasting effects of MDMA on the human brain. In animals, long-lasting neurotoxicity of MDMA is manifested as a dose-related loss of 5-HT axons or as sustained reductions in 5-HT neuronal signaling (Capela et al, 2009; Wilson et al, 1989; Fischer et al, 1995; Hatzidimitriou et al, 1999; Green et al, 2003; Lyles and Cadet, 2003), although evidence for axon loss is not found in all studies (Baumann et al, 2007; Wang et al, 2007; Fantegrossi et al, 2004). Human MDMA users have 5-HT alterations consistent with the animal neurotoxicity data that include reduced 5-HT transporter binding (McCann et al, 2008; Kish et al, 2010), increased 5-HT2A receptors (Reneman et al, 2002), and reduced 5-HT metabolite levels in cerebrospinal fluid (Ricaurte et al, 1990). Recreational MDMA users also have chronic neurocognitive (Kalechstein et al, 2007) and neuropsychiatric (Karlsen et al, 2008) impairments.
Despite this evidence for long-lasting brain effects, functional neuroimaging studies of human MDMA users have not found a consistent pattern of altered brain neurophysiology in association with MDMA exposure (Cowan, 2007; Cowan et al, 2008a; Daumann et al, 2003, 2004, 2005; Jager et al, 2008a, 2008b; Jacobsen et al, 2004; Moeller et al, 2004; Roberts et al, 2009; Raj et al, 2009). Several factors, including dose–response effects, polydrug effects, and task design, may account for the absence of a consistent neurophysiological effect of MDMA exposure in extant functional neuroimaging studies.
Several lines of evidence suggest that the visual system may be ideal as a paradigm system for revealing chronic MDMA effects. First, 5-HT axons densely innervate structures in the visual pathway, including the lateral geniculate nucleus (LGN) of the thalamus and the occipital cortex (Waterhouse et al, 1990), which contains the primary and secondary visual cortical regions (Tork, 1990). Second, MDMA administration produces long-lasting or permanent loss of 5-HT axon labeling in primate occipital cortex (Hatzidimitriou et al, 1999), which is prominent in distal occipital regions. Third, 5-HT influences visual cortical excitatory and inhibitory tone (Moreau et al, 2010), suggesting that MDMA-induced loss or reduction in 5-HT signaling would be expected to alter visual cortical excitability. Fourth, human MDMA use is associated with reduced occipital cortex 5-HT reuptake transporter (eg, McCann et al, 1998, 2005; Semple et al, 1999; Kish et al, 2010), increased 5-HT2A receptor binding (Reneman et al, 2002; reviewed by Cowan, 2007), and altered visual activation (Cowan et al, 2006). Fifth, MDMA users have altered visual perception (Brown et al, 2007; Oliveri and Calvo, 2003).
Given the lack of clarity in the human literature with regard to the persistent neurophysiological effects of MDMA on brain function, we designed a study to address unresolved questions with regard to MDMA's effects in human recreational users. We used visual stimulation in conjunction with the functional magnetic resonance imaging (fMRI) blood oxygenation level-dependent (BOLD) method to examine visual stimulus-evoked activation in the LGN and visual cortical subregions in Brodmann Area (BA) 17 and BA 18 in abstinent MDMA users and non-MDMA user control subjects. On the basis of previous MDMA research and the known neurophysiological role of 5-HT in visual regions, our primary hypothesis was that greater lifetime MDMA exposure would predict greater visual system stimulus-evoked signal intensity and spatial extent of activation that is not due to other drug effects and that does not resolve with abstinence. If these hypotheses are supported, the results would suggest that MDMA-associated toxicity leads to an increase in cortical excitability, likely due in part to altered 5-HT function.
MATERIALS AND METHODS
This study used a mixed, within-subject and between-groups design to assess the association of MDMA use with brain activation in the LGN of the thalamus and BA 17 and BA 18 of cerebral occipital cortex during visual stimulation in abstinent MDMA users and non-MDMA (control) subjects.
Subjects
Forty-five subjects took part in the visual stimulation paradigm: data from five subjects were excluded for technical failure. Research subjects were men or women, 18–35 years old, and reported previous MDMA use (MDMA users group; N=20) or other recreational drug or alcohol use (non-MDMA user controls; N=20). Subjects provided written informed consent and the protocol was approved by the Vanderbilt University Institutional Review Board and conformed to principles of the World Medical Association's Declaration of Helsinki.
Subjects enrolled in this study took part in a multimodal imaging study of MDMA effects, but did not overlap with those in our previous visual system study (Cowan et al, 2006). Some participated in our study of motor function (Karageorgiou et al, 2009). Subjects were screened with: clinician version of the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al, 1997) or Mini-International Neuropsychiatric Interview (Sheehan et al, 1998) urine drug testing (Triage Drugs of Abuse Panel, Biosite Diagnostics, San Diego, CA) and, if female, pregnancy screening (Sure-Vue Urine hCG, Fisher HealthCare, Houston, TX). Subjects were re-tested for drugs and pregnancy, and also passed an alcohol breathalyzer test (Alco Sensor III, Intoximeters, St Louis, MO) to confirm recent abstinence (see Abstinence Criteria) on each study day.
Subjects reported abstinence of at least 14 days for drug use and 48 h for alcohol use. Exclusions were as follows: general medical conditions, contraindications to MRI scanning, pregnancy, lifetime history of Axis-I psychiatric diagnoses, except for substance use/abuse/dependence-related disorders, current substance dependence (other than nicotine and caffeine), positive urine drug or alcohol screen, use of psychoactive or vasoactive medications within 6 weeks of enrollment, and head injury with loss of consciousness greater than 30 min.
Drug Use History Assessment
We assessed the extent of self-reported substance use with a drug use history questionnaire, utilizing a time-line follow-back method (Fals-Stewart et al, 2000). The questionnaire contains items to indicate when a specific drug was last used and assays recent and remote exposures as number of episodes and approximate amount of the drug used (Cowan et al, 2006, 2007). An episode of drug use was defined as use of a form of a drug (eg, snorting, injecting) within a single 24-h period. We relied on lifetime measures of drug use for our analyses because: (1) our primary aim was to assess for chronic cumulative MDMA effects; (2) the average duration of MDMA abstinence was over 1 year (therefore many subjects did not have past year or past month use); and (3) our previous studies have found that lifetime MDMA use is most strongly associated with brain activation outcomes.
Experimental Paradigm
We used a modified two-color visual stimulation paradigm that has proven sensitive in our previous studies to the effects of gender (Cowan et al, 2000), MDMA use (Cowan et al, 2006), and amphetamine administration (Cowan et al, 2008b) on occipital cortical BOLD fMRI signal. Subjects viewed red and blue light stimuli at a flash rate of 8 Hz at three intensity levels (low, medium, and high, based on the dynamic range of the computer display) with within-run randomization of color and intensity conditions. We chose a block design to maximize our ability to detect activation differences (Birn et al, 2002). We used three intensity levels to avoid floor or ceiling effects on activation. Each run contained four repetitions of each color and intensity. The duration of each light block was 10 s and each baseline period (black screen) was 14 s. Visual stimuli were generated using Matlab (Mathworks, Natick, MA) on a Macintosh computer. Stimuli were displayed using either liquid crystal diode (Resonance Technologies) goggles (for studies performed on the GE scanner) or onto a screen behind the subject's head using an Avotec projector and were viewed using a mirror placed within the bore of the scanner (for studies performed on the Philips scanner).
fMRI Data Collection
Thirty-one subjects were scanned with a Philips 3 T Intera Achieva MRI Scanner (Philips Medical Systems, Andover, MA) and nine subjects with a 3 T GE Signa whole body scanner (GE Healthcare, Milwaukee, WI). The following parameters were common to both scanners: 288 collected dynamics within a functional run between 582 and 592 s, 19 or 28 slices either 4.5 or 5 mm thick with a gap from 0.00 to 0.45 mm; TR=2 s, TE=35 ms; 70° or 79° flip angle; and FOV=240 × 240, matrix=128 × 128 pixels. Functional runs were co-registered to T1-weighted 3D anatomical images, acquired under the following parameters: 128 or 170 slices, 1.00 or 1.20 mm slice thickness, gap=0 mm; TE=3.7–4.6 ms, TR=7.9–8.9 ms; 2°–8° flip angle; and matrix=256 × 256 pixels, FOV=240 × 170 mm, or 256 × 170 mm. We assessed for potential differential scanner/delivery method effects using multiple approaches (see Online Supplement, Supplementary Table S1, and Supplementary Figure S1).
fMRI Data Analysis
Images were preprocessed and analyzed using in-house software and SPM5 (Wellcome Department of Cognitive Neuroscience, London, UK). Functional and anatomical data were co-registered and images were normalized to the SPM T1 template image, and then smoothed with a full-width half maximum 8-mm Gaussian kernel.
First-Level Analysis
We used a mass univariate approach as implemented in SPM5 to create individual voxel-level t-maps for stimulus-induced activation for each stimulus condition (red or blue color at low, medium, or high intensity) for each subject. T-maps generated in the first-level analysis were used for second-level analyses. In addition, the stimulus-evoked signal intensity data was extracted for additional analyses outside SPM5.
Second-Level Analysis
On the basis of the known anatomy of the visual system and on findings from our earlier studies, we examined stimulus-evoked activation in six a priori ROIs (right and left LGN; right and left BA 17; and right and left BA 18). We used the Wake Forest University Pickatlas tool (v.2.4) in SPM (Maldjian et al, 2003; Tzourio-Mazoyer et al, 2002) to dilate the LGN mask by one voxel in all dimensions to account for potential individual variations in this small structure. We transposed these six bilateral ROIs into cluster images using the MarsBaR ‘build' function. We used Monte Carlo simulation as implemented in AlphaSim (AFNI, NIMH) to determine a corrected statistical threshold for activation to visual stimulation in the a priori ROIs. To avoid partial volume effects arising from averaging physiologically non-relevant brain regions within the full anatomical BA 17 and BA 18 ROIs with those activated by the visual stimulus, we defined the stimulus-relevant portion of the ROI for BA 17 and BA 18 as those voxels activated during combined red and blue high stimulus intensity across both MDMA users and controls at a voxel-level p<0.05, with an extent threshold of 90 voxels (as determined using AlphaSim) for family-wise corrected p<0.05. We used these physiological ROI masks for bilateral BA 17 and BA 18 for subsequent analyses of signal intensity and spatial extent of activated voxels by extracting this information and exporting individual results to SPSS for further analysis. We used a threshold of p<0.001 uncorrected for multiple comparisons in the small volumes to determine the spatial extent of activated voxels in the physiological ROIs within BA 17 and BA 18. Because there were no activated voxels detected in the LGN using the corrected thresholds that were applied to measure BA 17 and BA 18 activation, we did not analyze the spatial extent of activation within the LGN and extracted only the signal intensity measure. We used an in-house MATLAB script to extract ROI data on a voxel level (to determine the proportion of activated voxels to generate the spatial extent measure) and MarsBaR to extract the signal intensity data.
Statistical Analysis
Descriptive statistics were used to summarize and initially inspect the distributions of demographic and study measures. Because MDMA and other drug use were non-normally distributed, we employed non-parametric approaches for within- and between-group comparisons for activation outcome measures.
Within-group analysis. Because our earlier fMRI studies (Cowan et al, 2006; Karageorgiou et al, 2009; Raj et al, 2009) revealed that lifetime MDMA exposure correlated with task-evoked brain activation across cohorts, tasks, and brain regions, our primary hypothesis was that lifetime MDMA exposure within the MDMA user group would predict visual system activation. Therefore, our primary analytic approach was the assessment of the relationships of stimulus-evoked signal changes with lifetime MDMA use (Spearman's rank correlations in SPSS; Spearman's rho; rs). We examined activation effects of the visual stimulation paradigm by measuring stimulus-evoked BOLD signal intensity and the spatial extent of stimulus-induced activation.
Between-group analysis. We compared between-group activation in the six a priori anatomical ROIs (right and left LGN, BA 17, and BA 18) within SPM using the contrast of red and blue high-intensity stimulus vs baseline. In addition, we used in-house Matlab scripts to extract the ROI data for stimulus-evoked BOLD signal change and compared mean signal intensity data between groups using Mann–Whitney tests in SPSS.
Outcome Measures
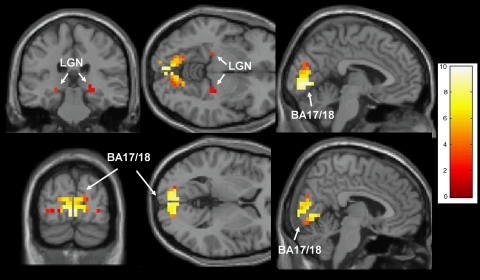
We examined patterns of activation from the second-level analyses for each color and stimulus intensity. Because the overall intensity of the average signal change was small and there were no clearly observed color or hemisphere-specific differences in the activation patterns, we chose initially to aggregate activation outcome data from the right and left hemispheres and for red and blue color. An analysis of the relationship of stimulus intensity from the aggregated data (low, medium, or high) with BOLD signal intensity in LGN, BA 17, and BA 18 revealed that BOLD signal intensity increased with increasing stimulus intensity with no evidence for a ceiling effect (Figure 1). The second-level analyses revealed also that the most extensive activation was produced by the combination of red and blue high-intensity stimuli. Therefore, all subsequent analyses were conducted using activation data (as BOLD signal intensity or spatial extent of activation) averaging signal obtained in red and blue high stimulus intensity conditions and from right and left hemispheres. Figure 2 shows the resulting pattern of activation in LGN, BA 17, and BA 18 following high-intensity visual stimulation. Activated voxels were not detected in the LGN using corrected statistical thresholds, but activation was robust in BA 17 and BA 18. Therefore (as noted above), signal intensity was used as the outcome measure for analyses in the LGN, whereas both signal intensity and spatial extent of activation were used as outcomes for BA 17 and BA 18.
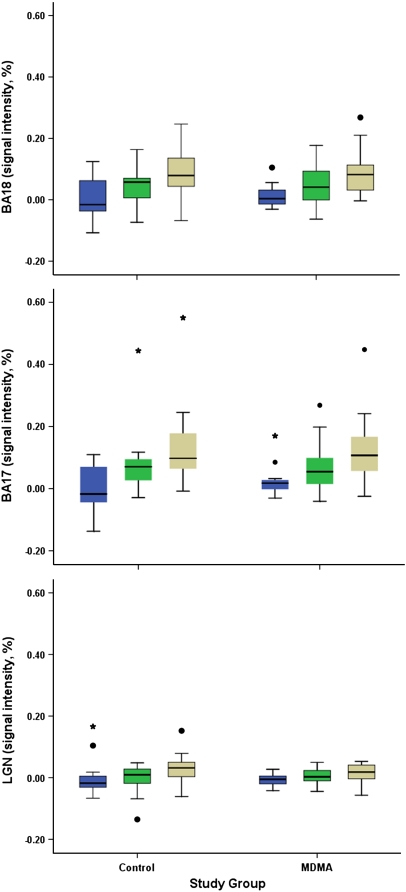
Figure 1.
Blood oxygen level-dependent (BOLD) signal intensity in lateral geniculate nucleus (LGN), Brodmann Area (BA) 17, and BA 18 in 3,4-methylenedioxymethamphetamine (MDMA) users and controls. BOLD signal intensity increased in all regions and in both groups with increasing visual stimulus intensity. Circles on box plot indicate outliers extending 1.5 times the box height; asterisks indicate extreme outliers extending three times the box height). Legend equals visual stimulus intensity:  , low;
, low;  , medium; and
, medium; and  , high.
, high.
Figure 2.
Activation to high-intensity visual stimulus. This figure depicts (left to right) coronal, axial, and parasagittal activation maps from all subjects (3,4-methylenedioxymethamphetamine (MDMA) and control) during high-intensity stimulation (combined red and blue light) overlayed on canonical brain T1 template from SPM5. Representative sections depict lateral geniculate nucleus (LGN) and Brodmann Areas (BA) 17 and 18. For display purposes, threshold is set at p<0.05 (t=1.65) uncorrected without a cluster extent threshold (one-sample t-test). This threshold was chosen for display because LGN activation was not detectable using the corrected cluster extent (a voxel level threshold of p=0.05 combined with a cluster extent of 90 voxels produced a family-wise error corrected p=0.05 for the total volume included in bilateral LGN, BA 17, and BA 18). Color bar indicates t-scores.
RESULTS
Sample Characteristics
Sample characteristics are summarized in Table 1. No statistically significant differences in age or gender distributions were found between the MDMA and non-MDMA user groups (p>0.05, Mann–Whitney and χ2 tests). No statistically significant associations of age or sex with any activation measure within any region were found (p>0.05, Spearman's rank correlations). Additional sample characteristics are provided in Online Supplement in Section ‘Additional Sample Characteristics'.
Table 1. Drug Use History.
|
MDMA (N=20) |
Non-MDMA (N=20) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Minimum | Maximum | Mean (SD) | Abstinence (days), mean (SD) | N | Minimum | Maximum | Mean (SD) | Abstinence (days), mean (SD) | |
| MDMA | ||||||||||
| Episodes | 20 | 3 | 155 | 33.25 (37.79) | 479.95 (580.65) | 0 | ||||
| Milligrams | 300 | 10 000 | 2692.38 (2712.01) | |||||||
| Alcohola | ||||||||||
| Episodes | 17 | 23 | 3851 | 653.85 (979.40) | 14.85 (8.41) | 19 | 4 | 2620 | 659.37 (888.48) | 36 (57.23) |
| Units | 91 | 23 754 | 3481.66 (6289.04) | 4 | 11 567 | 2281.71 (3355.99) | ||||
| Cannabis | ||||||||||
| Episodes | 16 | 9 | 2000 | 644.76 (727.62) | 142.65 (339.71) | 15 | 0 | 1599 | 124.03 (352.78) | 217.87 (244.69) |
| Joints | 9 | 4000 | 854.66 (1161.18) | 0 | 1063 | 81 (234.64) | ||||
| Cocaine | ||||||||||
| Episodes | 17 | 0 | 40 | 9.65 (11.24) | 449.82 (587.71) | 0 | ||||
| Grams | 0 | 46 | 6.16 (10.55) | |||||||
| METH | ||||||||||
| Episodes | 7 | 0 | 166 | 19.65 (45.23) | 1092.33 (1482.18) | 0 | ||||
| Milligrams | 0 | 8110 | 850.51 (2073.08) | |||||||
| Sedatives | ||||||||||
| Episodes | 12 | 0 | 80 | 18.40 (26.84) | 2 | 0 | 10 | 0.80 (2.55) | 509.00 (577.00) | |
| Milligrams | 0 | 800 | 177.75 (368.29) | 0 | 100 | 8.00 (25.46) | ||||
| LSD | ||||||||||
| Episodes | 16 | 0 | 148 | 18.20 (33.71) | 982.93 (692.81) | 1 | 0 | 15 | 0.75 (3.35) | 3173 (—) |
| Milligrams | 0 | 19 940 | 3243.75 (5458.40) | 0 | 1800 | 90.00 (402.49) | ||||
| Psilocybin | ||||||||||
| Episodes | 15 | 0 | 25 | 6.80 (6.89) | 573.92 (671.04) | 4 | 0 | 6 | 0.60 (1.57) | 1114.75 (1660.75) |
| Grams | 0 | 100 | 20.58 (27.17) | 0 | 12 | 1.11 (3.14) | ||||
| Codeine | ||||||||||
| Episodes | 10 | 0 | 20 | 3.30 (5.61) | 510.78 (452.22) | 1 | 0 | 30 | 1.5 (6.71) | 1860 (—) |
| Milligrams | 0 | 800 | 120.55 (211.82) | 0 | 300 | 15 (67.08) | ||||
| Opium | ||||||||||
| Episodes | 13 | 0 | 106 | 10.20 (23.22) | 881.42 (1028.04) | 0 | ||||
| Milligrams | 0 | 20 000 | 1305.15 (4438.52) | |||||||
Alcohol units: 1 unit=8 oz beer=1 glass wine=1 mixed drink.
Within-Group Effects
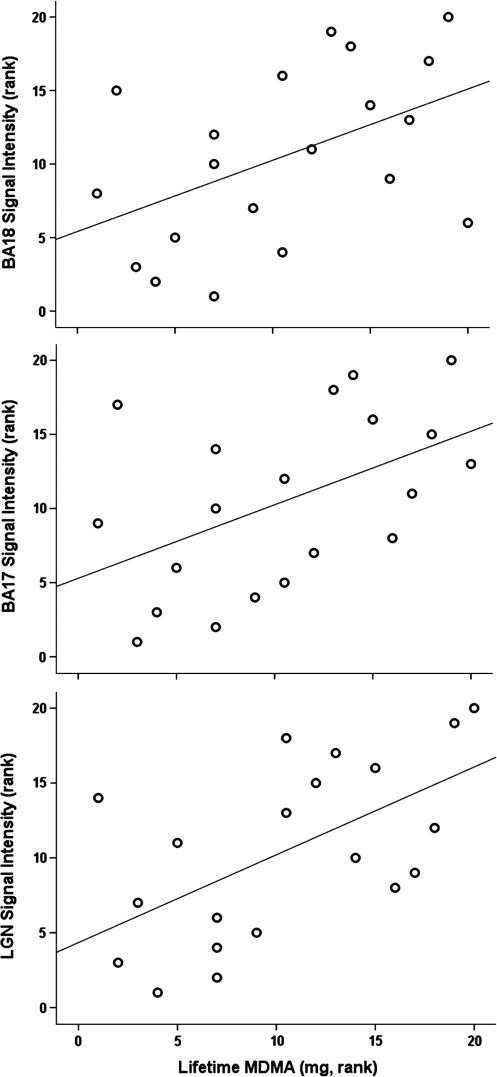
Effect of MDMA. Activation produced by the high-intensity stimulation is shown in Figure 2. The major finding from the within-subject correlation analysis was that greater lifetime MDMA use was statistically significantly associated with greater stimulus-evoked visual activation for signal intensity in LGN, BA 17, and BA 18 (Table 2; Figure 3), and for spatial extent of activation in BA 17 and BA 18 (Table 2) (p>0.05, Spearman's Rank correlations). There was no statistically significant association of the duration of MDMA abstinence with any outcome measure for any ROI.
Table 2. Within-Group Drug Effects.
| Lifetime drug | N |
Activation measure |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Signal intensity |
Spatial extent |
||||||||||
|
LGN |
BA 17 |
BA 18 |
BA 17 |
BA 18 |
|||||||
| rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | rs | p-Value | ||
| MDMA (mg) | 20 | 0.59a | 0.007 | 0.50 | 0.027 | 0.48 | 0.031 | 0.59 | 0.007 | 0.55 | 0.013 |
| MDMA+METH (mg)b | 20 | 0.57 | 0.009 | 0.59 | 0.006 | 0.60 | 0.006 | 0.69 | 0.001 | 0.65 | 0.002 |
Abbreviations: BA, Brodmann Area; LGN, lateral geniculate nucleus; METH, methamphetamine.
Significant correlations (rs=Spearman's ρ p<0.05) are shown in bolded text.
MDMA+METH=lifetime mg of MDMA plus lifetime mg METH use for the seven subjects reporting use of both drugs.
Figure 3.
Relationship of lifetime 3,4-methylenedioxymethamphetamine (MDMA) use to signal intensity in lateral geniculate nucleus (LGN), Brodmann Area (BA) 17, and BA 18. Y axis is the rank of signal intensity for the ROI (calculated as mean percent BOLD signal increase for high-intensity stimuli in right and left hemisphere). X axis is the rank of self-reported lifetime MDMA use in mg. Data shown for MDMA users only, N=20.
Effect of combined MDMA and methamphetamine. Of the 20 MDMA subjects, seven also reported use of methamphetamine (METH). Because METH produces serotonergic toxicity similar to that caused by MDMA (Peat et al, 1983; Woolverton et al, 1989), we reasoned that both drugs might produce similar and therefore additive effects on brain activation, especially if the observed findings are due to altered 5-HT neurophysiology. To explore this possibility, we created a measure of substituted amphetamine exposure that combined MDMA and METH exposures. As we did not have a priori data to suggest the mg equivalent 5-HT toxicity produced by each drug in human beings, we simply summed the lifetime quantity of MDMA and METH use (in mg), shown as MDMA+METH in Table 2. Overall, the composite measure accounted for 32% of the variance (as the square of rs) for LGN signal intensity vs 35% for MDMA alone. For BA 17 signal intensity, the composite measure accounted for 35, vs 25% of the variance for MDMA alone, with similar results found for BA 17 spatial extent, and for both signal intensity and spatial extent in BA 18. As for MDMA, there was no statistically significant association of the duration of METH abstinence with any activation measure in the METH-exposed subgroup.
Other drug effects. Although our primary analysis revealed that greater lifetime MDMA use, and greater lifetime use of MDMA+METH was associated with greater activation in all visual regions, MDMA users in our cohort (consistent with worldwide use patterns) reported exposure to multiple recreational drugs other than MDMA. We therefore examined the data for the influence of other drugs. Because partial correlations require data for all three variables in a specific analysis (activation measure, MDMA use, specific other drug use) and to maintain a sample of size of at least 10, these analyses were conducted only for those drugs used by at least 50% of the MDMA cohort (Table 1; alcohol, cannabis, cocaine, codeine, LSD, opium, psilocybin, and sedatives). There were no statistically significant associations of lifetime quantity of drug use with activation outcome variables (Spearman's rank correlation; p>0.05).
Effects of Scanner/Stimulus Delivery Method
As noted in the Materials and Methods, we acquired data on two different scanners, each employing a different stimulus delivery method (goggle vs projection screen). It is theoretically possible that the use of the different scanner/stimulus delivery methods might have affected our results. We conducted an analysis of the potential effects of these differences on our findings.
Lifetime quantity of MDMA use by scanner/stimulus method. MDMA use levels showed mean differences and correlations with scanner type at a trend (p<0.10 level). Mean MDMA use was slightly greater for MDMA users studied on the GE scanner (p=0.076; Mann–Whitney) (Supplementary Table S1). Scanner type was nonsignificantly correlated with lifetime quantity of MDMA use in mg (rs=0.41, p=0.074; Spearman's rank correlation).
Relationship of scanner to correlation of lifetime MDMA use with signal intensity. Supplementary Figure S1 depicts scatter plots of data from GE and Philips scanners to show the degree of overlap and pattern of data from the two scanners. The MDMA users studied on the GE scanner had higher minimum use levels than the Philips group, but there was good overlap for use levels in the higher use ranges.
Partial correlation analysis controlling for scanner effects. Although the graphic analysis suggests no scanner/stimulus-specific differences in slope of the relationship, we conducted a statistical analysis of the data adjusting for scanner/stimulus condition. We anticipated that as MDMA use was correlated at a trend level with scanner/stimulus method and as the additional scanner covariate would reduce the available degrees of freedom, a partial correlation analysis adjusted for scanner/stimulus would yield a weaker relationship between MDMA use and regional signal intensity. However, despite these factors, the partial correlation of lifetime quantity of MDMA use with regional visual stimulus-evoked signal intensity remained positive, but significantly so only for LGN (LGN, r=0.60, p=0.006; BA 17, r=0.35, p=0.092; and BA 18, r=0.351, p=0.141).
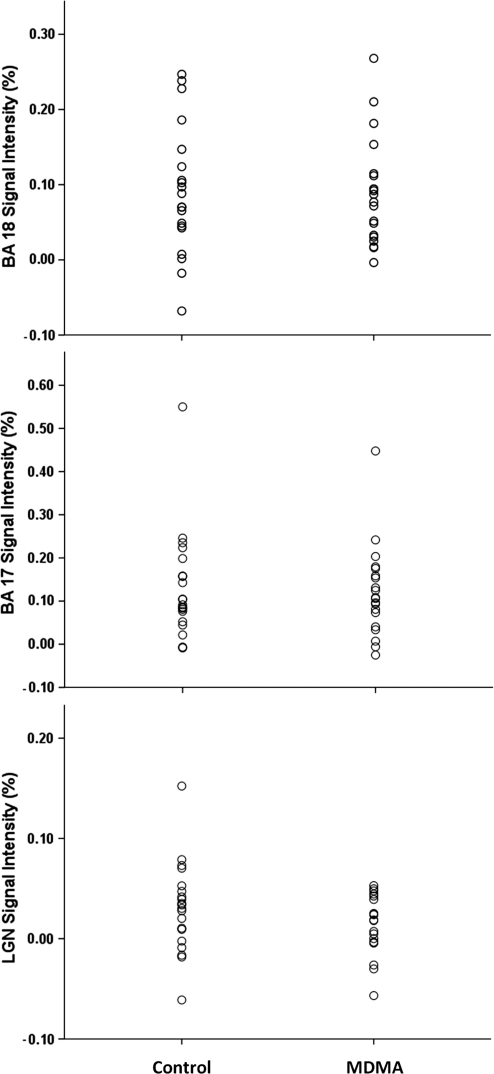
Between-Group Comparison
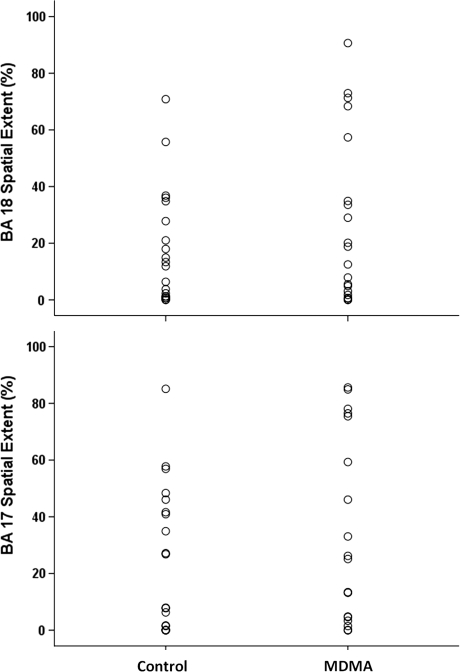
As predicted by data from our earlier study, there was general overlap between control group and MDMA user group activation measures, and there were no statistically significant differences between the MDMA and control groups in average signal intensity within the LGN, BA 17, or BA 18 (Table 3; Figure 4). In addition, there were no statistically significant between-group differences in the spatial extent of activation for BA 17 and BA 18 (Table 3; Figure 5). As noted above, spatial extent analysis was not performed for the LGN.
Table 3. Between-Groups Comparison.
| ROI |
Group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Measurea | MDMA (mean±SD) | Non-MDMA (mean±SD) | p-Value* | High-MDMA (mean±SD) | p-Value** | Low-MDMA (mean±SD) | p-Value*** | |
| LGN | Signal intensity | 0.014±0.030 | 0.031±0.045 | 0.221 | 0.033±0.016 | 0.713 | −0.006±0.027 | 0.015 |
| BA 17 | Signal intensity | 0.121±0.104 | 0.132±0.124 | 0.989 | 0.170±0.114 | 0.248 | 0.068±0.022 | 0.248 |
| Spatial extent | 32.210±32.48 | 24.53±25.63 | 0.478 | 51.899±33.491 | 0.031b | 12.529±15.633 | 0.328 | |
| BA 18 | Signal intensity | 0.089±0.070 | 0.091±0.085 | 0.904 | 0.1204±0.082 | 0.475 | 0.057±0.038 | 0.350 |
| Spatial extent | 26.685±29.533 | 17.863±20.200 | 0.529 | 43.227±33.174 | 0.049 | 10.143±11.520 | 0.373 | |
Abbreviations: BA, Brodmann Area; LGN, lateral geniculate nucleus; ROI, region of interest; SD, standard deviation of the mean.
Data are presented as mean percent signal intensity or spatial extent (activated voxels) in an ROI. Spatial extent data is not reported for LGN because no activated voxels were detected in LGN using corrected statistical thresholds. Between-group comparisons were performed using Mann–Whitney U-test for non-parametric data.
Bolded text, significant at p<0.05.
*p-Value represents comparison of entire MDMA group to non-MDMA control group.
**p-Value represents comparison of high-exposure MDMA subgroup to non-MDMA control group.
***p-Value represents comparison of low-exposure MDMA subgroup to non-MDMA control group.
Figure 4.
Signal intensity by brain region and group. Plot of individual signal intensity values by group shows the degree of overlap between the control and 3,4-methylenedioxymethamphetamine (MDMA) groups. As shown in Table 2, mean signal intensity did not differ by group. BA, Brodmann Area; LGN, lateral geniculate nucleus.
Figure 5.
Spatial extent of activation by brain region and group. Plot of individual spatial extent of activation by group shows the degree of overlap between the Control and 3,4-methylenedioxymethamphetamine (MDMA) groups. As shown in Table 2, mean spatial extent of activation did not differ by group. Corrected threshold for activated voxels was p<0.05, cluster extent 90 voxels (one-sample t-test). Because there were no activated voxels in lateral geniculate nucleus (LGN) using corrected thresholds, spatial extent data is shown only for Brodmann Area (BA) 17 and BA 18.
Given the relationship between lifetime MDMA exposure and activation shown above for the within-group analysis, we anticipated that the lack of between-group differences may have been secondary to the fact that the MDMA user group was not homogenous—that is, individuals with greater MDMA exposure would have higher mean activation measures than those with lower exposure. To address this issue, we conducted a subgroup analysis using a median split procedure to divide the MDMA user group into high- and low-exposure groups. To aid in controlling for Type I error, we used a non-parametric ANOVA (Kruskal–Wallis), followed by a post hoc Mann–Whitney test. This analysis revealed that the high-exposure MDMA group had significantly greater spatial extent of activation when compared with the control group, but there were no significant between-group differences in signal intensity (Table 3). In contrast to the greater spatial extent of activation seen for the comparison of high-exposure MDMA users to the control group, the only significant difference between low-exposure MDMA users and control subjects was found in the LGN where the low-exposure MDMA subgroup had lower signal intensity than the controls (Table 3). Only the signal intensity difference in LGN met the adjusted α-level (p<0.017) after accounting for the number of comparisons.
DISCUSSION
The primary finding of this study is that greater lifetime MDMA exposure was associated with greater stimulus-evoked visual system activation and this relationship was not affected by the duration of MDMA abstinence. Greater cumulative lifetime MDMA use was associated with greater stimulus-evoked visual system BOLD signal intensity in the LGN, BA 17, and BA 18, and with greater spatial extent of activated voxels in BA 17 and BA 18. Although there were no between-group differences in activation outcome measures when the entire MDMA group was compared with the non-MDMA-exposed control group, subgroup analyses suggested that high-exposure MDMA users tended to have greater activation than controls, most notably for the spatial extent of activation. Additive effects of MDMA and METH on both signal intensity and spatial extent of activation in BA 17 and BA 18 were also observed, supporting the possibility that the 5-HT neurotoxicity common to both drugs may account in part for the observed results. However, this finding needs to be interpreted cautiously until replicated. The long average duration of self-reported MDMA abstinence and the positive exposure–activation relationship, despite a long average duration of abstinence, suggests that MDMA use may cause graded, long-lasting, and potentially permanent alterations in human visual system neurophysiology. The detected exposure-related increases in task- or stimulus-evoked activations may be a neurophysiological biomarker for MDMA neurotoxicity.
Relationship to Previous Findings
As reviewed previously (Cowan, 2007; Cowan et al, 2008a), previous studies employing more complex cognitive paradigms found a range of activation patterns following chronic MDMA exposure (Daumann et al, 2003, 2004, 2005; Jager et al, 2008a, 2008b; Jacobsen et al, 2004; Moeller et al, 2004). Subsequent studies have revealed a similarly mixed picture when using fMRI to examine brain activation during cognitive paradigms (Roberts et al, 2009). Our own recent report of semantic processing showed a largely inverse association of lifetime MDMA use and signal intensity, but this inverse association was found after a complex subtraction analysis of word and non-word tasks in regions weakly activated by the employed paradigm (Raj et al, 2009). Several behavioral and pharmacological aspects of recreational MDMA use likely account for the lack of consistent findings with regard to MDMA effects on brain neurophysiology in previous reports. First, MDMA toxicity in animals is dose-dependent (Green et al, 2003), suggesting that human MDMA imaging research study design might need to account for individual variation in drug exposure. Second, MDMA users worldwide have high levels of exposure to other drugs (Wish et al, 2006), which may thwart attempts to specifically associate MDMA use with altered brain function. These polydrug effects may be accounted for in part by comparison with control groups having other drug (but no MDMA) exposure and several investigators have attempted to include drug-matched controls in their study design. However, matching average use across groups does not account fully for individual patterns in drug exposure and also controls only for the most commonly used substances (e.g., cannabis). Third, most studies investigating MDMA effects on human neurophysiology have employed paradigms that involve the performance of complex cognitive tasks during functional neuroimaging (reviewed by Cowan, 2007). However, interpreting functional neuroimaging findings arising from complex cognitive tasks in terms of underlying brain neurophysiology is challenging. Cognitive processing is more widely distributed in the brain than simple sensory or motor function, and brain activation results from cognitive paradigms are often reported as contrasts of tasks, potentially obscuring relationships to more basic neurophysiology (reviewed by Cowan, 2007). Our experimental approach was specifically designed to attempt to account for these factors.
In this study and in our earlier report of visual system function in MDMA users (Cowan et al, 2006), we did not find between-group differences in average stimulus-evoked activation when MDMA users were compared with non-MDMA-exposed control subjects. However, in both our initial and current reports, we found that the MDMA user subgroup having the greatest lifetime MDMA exposure showed a greater spatial extent of activation than non-MDMA-exposed controls. This suggests that studies restricting enrollment to individuals with heavier MDMA use would be more likely to find greater activation in the MDMA user group, whereas studies enrolling MDMA users with lower MDMA exposure or a broad range of MDMA exposure might find opposite or no between-group differences. It is also possible that the effect observed here for the visual system is not present in all cortical regions, even for simple sensory tasks. However, an evoked potential study of 5-HT-related auditory processing found, similarly to our fMRI findings, that lifetime MDMA use was associated with greater amplitude of cortical response to auditory stimuli (Croft et al, 2001).
The absence of strong between-group differences in activation does not suggest that MDMA is safe or that there are no differences in brain function in MDMA users and controls. The increased spatial extent of activation in the higher-use MDMA group suggests that between-group differences emerge with greater MDMA exposure levels, which is consistent with the strong exposure–response effect shown in the within-group analysis for MDMA. Two additional factors may account for the lack of strong differences in the between-group comparison. First, MDMA has multiple mechanisms of potential neurotoxicity. Each of these may manifest differently in terms of neurophysiological effects as seen in the fMRI signal, with effects of 5-HT axon loss dominating at higher MDMA dosages. Second, other drugs may also be associated with reductions in the fMRI signal (Raj et al, 2009) and the effects of 5-HT axon loss may therefore be obscured in MDMA users with very low MDMA exposures. This is a pattern consistent with the clear exposure–response relationship shown within the MDMA user group.
Our study used the fMRI BOLD method to assay for MDMA effects on brain physiology. The fMRI BOLD method indirectly assays brain neurophysiology because increased focal neuronal synaptic or metabolic activity is associated with increased local blood flow (reviewed by Ekstrom, 2010). Increased task- or stimulus-evoked regional blood flow above a defined baseline is considered ‘activation' of a brain region and activation can be reported as the magnitude of BOLD signal intensity increase above baseline (a continuous measure of the signal change) or as the number, volume, or spatial extent of activated voxels (a discrete measure of voxels in which signal change exceeds a defined statistical threshold). Using these metrics, our initial report (Cowan et al, 2006), in a cohort not overlapping with the current group, suggested that greater MDMA use was associated with greater spatial extent of occipital activation. However, this early study was not designed to account for cumulative MDMA dosing or to control for the duration of MDMA abstinence or for polydrug effects, and methodological limitations of the scanner technology prevented the isolation of effects in specific anatomical regions. These results replicate the initial finding with regard to spatial activation, but not with regard to signal intensity. We suspected that the failure to detect an association of MDMA use and signal intensity in the original study was likely due to partial volume effects of averaging a small area of physiologically relevant task-evoked signal across a broader anatomically relevant region of interest. We extend that finding here by: (1) revealing that greater MDMA use is associated with not only greater spatial extent of activation, but also with greater signal intensity, and (2) by showing alterations in visual thalamus (LGN) and specific occipital cortical subregions, BA 17 and BA 18. These visual pathway findings, when coupled with evidence from our recent fMRI report of MDMA effects during motor task performance (Karageorgiou et al, 2009), show that simple sensory or motor fMRI paradigms are sensitive assays of potential serotonergic neurotoxicity associated with MDMA use.
We found that an aggregate measure combining lifetime MDMA exposure with lifetime METH exposure accounted for a greater proportion of the variance in activation in visual cortical regions (BA 17 and BA 18) than for MDMA exposure alone. Although METH effects on a simple visual fMRI paradigm have not been previously reported to our knowledge, several studies of brain activation during cognitive paradigms in METH-dependent individuals have found a mixed pattern of activation, similar to that observed in MDMA users, when compared with control subjects during a facial affect matching task (Payer et al, 2008) and a delay discounting task (Monterosso et al, 2007; Hoffman et al, 2008).
Specificity to 5-HT
Associations of MDMA use with altered activation measures in these data do not prove that MDMA exposure altered 5-HT function or caused 5-HT axon loss. However, several indirect lines of evidence suggest that serotonergic toxicity likely explains in part the observed findings. First, animal models have repeatedly revealed that MDMA exerts a dose-related toxicity to serotonergic axons (reviewed by Green et al, 2003) and our data show a similar dose–response effect on activation measures. Second, although MDMA produces neuroplastic changes as altered spines or dendrite shape in medial prefrontal cortex and nucleus accumbens in rats (Ball et al, 2009), and may induce scattered neuronal cell death (Schmued, 2003), we are not aware of evidence to suggest that these effects are prominent in visual system regions. Third, as discussed in the section that follows, 5-HT plays a major role in excitation/inhibition balance in the cortex (Moreau et al, 2010), and specifically with respect to cortical pyramidal response to thalamic inputs in visual system where serotonin limits the lateral spread of incoming excitation. Finally, MDMA and METH produce similar effects on 5-HT axon markers (eg, Peat et al, 1983; Woolverton et al, 1989; reviewed by Quinton and Yamamoto, 2006), and the aggregate MDMA+METH measure explained a greater portion of the variance in activation in both visual cortical regions.
The Observed Findings May Reflect Altered Cortical Excitability
Several lines of evidence converge to suggest that the most prominent effect of MDMA-induced 5-HT axon loss may be cortical hyper-excitability. We have previously speculated that (Karageorgiou et al, 2009), as have others (Jacobson et al, 2005; Vannini et al, 2007), increased activation in the presence of normal task performance is consistent with reduced cortical efficiency. If interpreted in terms of the amount of signal change or the spatial extent in cortical or subcortical regions activated by the visual stimulus, these and earlier findings (Cowan et al, 2006; Karageorgiou et al, 2009) would be consistent with less efficient brain functioning in association with MDMA use, in that more neural work (as indirectly indexed by increased signal and therefore potential metabolic demand) and more neural volume (as indexed by the increased spatial extent of spread) are utilized for sensory response. In line with our initial visual study and this report, we also recently reported that a motor task produced greater activation in cortico-striato-thalamic loops as lifetime MDMA use increased (Karageorgiou et al, 2009). In aggregate, these studies suggest that greater task- or stimulus-evoked activation with greater MDMA exposure may be a general neurophysiological and dose-related consequence of recreational MDMA use in human beings, especially when assayed with relatively simple visual and motor activation paradigms. We now propose that the neural basis for the observed reduction in cortical efficiency (measured as increased activation) in association with MDMA use is due in part to an increase in cortical excitability that is secondary to MDMA-induced reduction in serotonin signaling. As discussed below, evidence consistent with MDMA-associated increases in cortical excitability has been reported from electrophysiological studies in mice and in human visual cortex. These results provide further neurophysiological support for MDMA-induced cortical, and possibly thalamic, hyper-excitability.
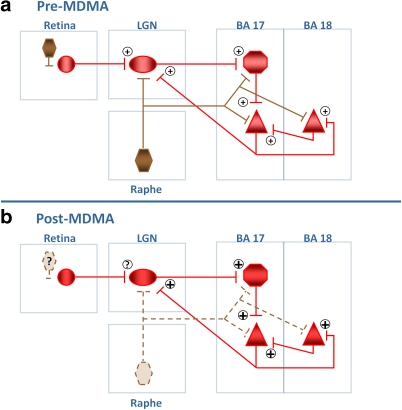
How might greater MDMA exposure lead to task- or stimulus-evoked cortical hyper-excitability? The origins of the fMRI BOLD signal are not fully understood, but the bulk of what is measured as ‘activation' in fMRI studies is thought to reflect indirectly graded synaptic inputs to a brain region and their consequences (measured as the local field potential) and local neuronal processing, determined in part by glutamate release (Logothetis, 2008; Bennett et al, 2008). However, the precise neural and metabolic events contributing to the BOLD signal are complex and these likely vary with local neural circuitry (reviewed by Ekstrom, 2010). Factoring in visual system neural circuitry and current knowledge of the BOLD signal in visual cortex, we have developed a tentative, highly simplified, neural model (Figure 6) to help explain the positive correlation of MDMA exposure and activation measures in visual system and as reflective of the net effect of MDMA exposure on LGN and cortical output (pyramidal) neurons. 5-HT influences retino-geniculate synaptic transmission in a frequency-dependent manner through multiple receptors and through pre- and post-synaptic mechanisms (Seeburg et al, 2004). Dorsal raphe (the source for the fine diameter 5-HT axons most vulnerable to substituted amphetamines) stimulation in rats leads to the inhibition of LGN response to retinal stimulation and decreased thalamo-cortical excitation (Yoshida et al, 1984). Therefore, loss or reduction of dorsal raphe 5-HT input to LGN may result in increased stimulus-evoked thalamo-cortical excitation. In neocortex, 5-HT receptors of multiple subtypes are present on thalamo-cortical afferents, excitatory, and inhibitory neurons. In pyramidal neurons, 5-HT is both excitatory (via 5-HT2A receptors on dendrites and spines) and inhibitory (via 5-HT1A receptors on the axon hillock) (Rauch et al, 2008). 5-HT1A agonists inhibit the lateral spread of cortical excitation, suggesting that loss of agonist signaling at these receptors might increase spatial spread of excitation in the cortex. This model reflects only the potential net effects of MDMA exposure and does not address cellular or receptor level alterations. MDMA has been associated with the loss of retinal ganglion and other retinal cells in animals (Miranda et al, 2007), but we do not include this information in the present model because the functional implications are unclear.
Figure 6.
3,4-Methylenedioxymethamphetamine (MDMA)-induced reduction in visual pathway serotonin (5-HT) signaling may produce shifts in cortical excitability. Figure depicts simplified visual pathway circuit in pre-MDMA and post-MDMA conditions. 5-HT is shown in brown; glutamatergic neurons are shown in red. Plus (+) sign indicates excitatory transmission. (a) Pre-MDMA: the retina contains 5-HT containing cells and glutamatergic retinal ganglion cells that project to lateral geniculate nucleus (LGN). LGN provides feedforward glutamatergic excitatory projects to Brodmann Area (BA) 17 glutamatergic stellate cells (red octagon), which (through additional local processing) leads to feedforward excitation to glutamatergic pyramidal neurons in BA 17 that provide feedforward excitation to BA 18 pyramidal (and other neurons). BA 17 pyramidal cells provide feedback excitatory projection to LGN (which may synapse on inhibitory interneurons). Raphe 5-HT inputs to LGN, BA 17, and BA 18 neurons modulate evoked neuronal activity with mixed inhibitory or excitatory actions depending upon neuron and receptor type. On pyramidal neurons, the dendritically located 5-HT2A receptor is excitatory, whereas the 5-HT1A receptor at the axon hillock is inhibitory (receptors not shown in diagram). (b) Post-MDMA: following MDMA exposure, 5-HT neurotransmission is reduced, either through frank axotomy or reduced 5-HT synthesis. Retinal effects of MDMA on 5-HT are unclear. The net effect of altered 5-HT input to LGN, BA 17, and BA 18 is depicted as increased excitation at each juncture. (Note that if BA 17 feedback to LGN results normally in inhibition of LGN projection neurons through inhibitory interneurons, then increased BA 17 excitatory output might lead to increased feedback inhibition to LGN, and thus reduce feedforward excitation to BA 17. On the basis of the available data, the net effect is depicted as overall increased excitation in the thalamo-cortical pathway.).
Directly relevant to this study, Oliveri and Calvo (2003) used transcranial magnetic stimulation to elicit visual phosphenes (which they defined as a conscious report of light sensation in the absence of a visual stimulus) in MDMA users and controls and found that the stimulus threshold for generating phosphenes was lower in MDMA users. Giorgi et al (2005) used a mouse model of MDMA administration and found that MDMA increased hippocampus and entorhinal cortex excitability as measured using electroencephalography, a kainate-induced seizure threshold determination paradigm, and glucose uptake. More globally, 5-HT plays a role in setting the gain for inputs to pyramidal neurons (Higgs et al, 2006) and blocks or reduces seizure activity in multiple seizure models (reviewed by Bagdy et al, 2007), further supporting an overall role for this neurotransmitter of reducing cortical excitability. If the BOLD signal largely reflects the consequences of glutamate signaling (Ku et al, 2008; Bennett et al, 2008), our data thus far are consistent with thalamo-cortical, cortical-cortical, and cortico-thalamic increases in glutamate release reflected in increased BOLD signal. As summarized in Figure 6, loss or reduction of dorsal raphe 5-HT input to LGN, BA 17, and BA 18 could result in increased LGN excitability to retinal ganglion cell input, as well as increased local excitatory (via recurrent excitation; Cowan and Wilson, 1994) and feedforward and feedback projections between LGN, BA 17, and BA 18. However, given the complexity of 5-HT innervation and action and the uncertainty of the origin of the BOLD signal, this interpretation is necessarily speculative and is presented mainly to aid understanding and as a framework for future studies aimed at refining this idea. Notably, factors other than MDMA-induced 5-HT neurotoxicity, including neuroplastic changes (Ball et al, 2009), focal neuronal death (Schmued, 2003), altered regional metabolite concentrations or brain gray matter density (Cowan et al, 2003, 2007, 2008a), or other neurotransmitter or neurotoxic effects (Yamamoto et al, 2010), could account in part or in total for the observed findings.
Limitations
Several factors limit our ability to draw definitive conclusions with regard to the role of MDMA in producing altered brain activation. A cross-sectional study design as employed here cannot assess causation. User self-report of previous MDMA exposure is subject to inaccuracies through recall bias, deception, forgetting, and impurity in Ecstasy pills sold as MDMA. Drug abstinence was verified only for the time period in which drugs or metabolites are detected by urine screens (2–3 days for most drugs), with the result that some subjects could have under-reported recent drug use. However, recent exposure to drugs other than MDMA seems unlikely to have produced the observed exposure–response association with lifetime MDMA use. We grouped data acquired using two scanning/stimulation methods; however, our analysis of the effect of scanner/stimulus on outcomes did not suggest that methodological factors influenced the results. In addition, these findings are consistent with our earlier report using a non-overlapping cohort of MDMA users studied using a 1.5 T scanner and different stimulus methods (Cowan et al, 2006), as well as with our recent motor system study performed in a partially overlapping cohort, but on a single scanner (Karageorgiou et al, 2009).
Because our hypotheses predicted that MDMA use would be associated with altered spatial extent of activation and to avoid partial volume effects, we devised an experimental approach that permitted us to isolate the physiologically relevant (task-relevant) sub-region in larger a priori anatomically defined areas (LGN, BA 17, and BA 18). An alternative approach has been used in retinotopic mapping methods to precisely isolate primary (V1) and secondary (V2) visual regions. However, the interpretation of retinotopic mapping, to our knowledge, is ambiguous in the presence of neurophysiological alterations that influence potentially both the probability of declaring a voxel as activated (via effects on increased signal intensity, and thus increased signal-to-noise ratio) and the spatial extent of activation (via effects on the spread of recurrent excitation and inhibition). And, it is not clear that knowing MDMA effects specifically in V1 and V2 would add to our understanding of the general effects of MDMA on cortical neurophysiology.
It is possible that pre-existing brain differences are responsible for the observed results, but we are not aware of a mechanism through which altered visual system excitability would predispose to higher levels of MDMA use. The observed fMRI findings cannot be assumed to imply altered conscious visual perception in this cohort and future studies using biophysical measures in conjunction with fMRI studies are necessary to address this issue. Taffe et al (2001, 2002, 2003) reported that MDMA administration regimens in monkeys that deplete cortical 5-HT by greater than 75% (measured in post-mortem assays) produced detectable behavioral deficits only during tryptophan depletion or during administration of drugs active at 5-HT receptors. These findings suggest that, despite chronic MDMA-induced reductions in brain 5-HT content, compensatory brain mechanisms may sustain brain functions in non-human primates.
However, we think that the significance of this report lies not in detecting visual system dysfunction per se, but more broadly in showing a potential chronic and widespread shift in brain excitability that may be a neural signature of MDMA toxicity. Functional MRI is an indirect method that is influenced both by neuronal and vascular function, and it is therefore possible that the observed effects may not be due to altered neuron to neuron communication. However, we believe this is unlikely because human MDMA use has not been associated with chronically altered cerebral blood flow (Chang et al, 2000). McCann and Ricaurte (2007) and McCann et al (2009a, 2009b) have found that MDMA users have altered sleep, including higher rates of sleep apnea and increased vulnerability of cognitive performance to the effects of sleep deprivation. We did not assay sleep parameters in this cohort, and therefore we cannot rule out the possibility that sleep deprivation may have influenced our results.
Conclusions
These results suggest that recreational MDMA exposure leads to chronic alterations in visual system neurophysiology. These results, and those of others using different techniques, indicate that the observed association of MDMA use with brain activation may be secondary to cortical hyper-excitability upon exposure to a sensory stimulus. Additional studies are needed to replicate this finding, to determine which additional cortical regions show this effect, whether stimulus intensity or task demands influence the observed pattern, and perhaps to show prospectively that MDMA exposure causes altered cortical neurophysiology. If the observed associations of MDMA use with increased activation result from shifts in cortical excitatory thresholds, the implications of this finding and the underlying neural basis for this phenomena need to be more precisely determined. Notably, our study excluded individuals who reported co-morbid psychiatric conditions such as major depression or anxiety disorders. As these disorders are linked to 5-HT function and are reported at higher rates in MDMA users, our study may have excluded a subgroup having greater vulnerability to MDMA toxicity. Additional studies specifically examining MDMA effects in subjects with co-morbid psychiatric conditions appear urgently warranted. Given the many millions of individuals worldwide who have used or continue to use MDMA and ongoing clinical trials investigating MDMA's therapeutic potential, large prospective studies of brain function in abstinent users seem essential to determine whether MDMA-associated effects remain stable, improve, or worsen over time.
Acknowledgments
This work was supported by NSF (IGERT DGE-0801634 to ALB), NIDA/NIH (R01 DA01537 and R21 DA020149 to RLC, and T32DA021123 to EJC), NIMH/NIH (K01 MH083052 to JUB), and NCRR/NIH (Vanderbilt CTSA Grant UL1 RR024975).
ALB, JUB, EJC JGL, CJC, NDW, AC, TW, CR D I, CC, and RMS report no potential conflicts of interest related to this report. MSD performed consultation or received compensation from the American Economic Association, Belmont University College of Health Sciences, and the University of Oklahoma Health Sciences Center. RLC received publication royalties from Lippincott Williams and Wilkins and consultant income from the Southwest Michigan First Life Science Fund, the University of West Alabama, Novo Nordisk, and Shire Pharmaceuticals.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. Origins of the BOLD changes due to synaptic activity at astrocytes abutting arteriolar smooth muscle. J Theor Biol. 2008;252:123–130. doi: 10.1016/j.jtbi.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection vs estimation in event-related fMRI: choosing the optimal stimulus timing. NeuroImage. 2002;15:252–264. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Brown J, Edwards M, McKone E, Ward J. A long-term ecstasy-related change in visual perception. Psychopharmacology (Berl) 2007;193:437–446. doi: 10.1007/s00213-007-0785-0. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Chang L, Grob CS, Ernst T, Itti L, Mishkin FS, Jose-Melchor R, et al. Effect of ecstasy [3,4-methylenedioxymethamphetamine (MDMA)] on cerebral blood flow: a co-registered SPECT and MRI study. Psychiatry Res. 2000;98:15–28. doi: 10.1016/s0925-4927(99)00048-7. [DOI] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology. 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Frederick BB, Rainey M, Levin JM, Maas LC, Bang J, et al. Sex differences in response to red and blue light in human primary visual cortex: a BOLD fMRI study. Psychiatry Res. 2000;100:129–138. doi: 10.1016/s0925-4927(00)00074-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Haga E, de Frederick BB, Dietrich MS, Vimal RL, Lukas SE, et al. MDMA use is associated with increased spatial BOLD fMRI visual cortex activation in human MDMA users. Pharmacol Biochem Behav. 2006;84:219–228. doi: 10.1016/j.pbb.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, et al. Reduced cortical gray matter density in human MDMA (Ecstasy) users: a voxel-based morphometry study. Drug Alcohol Depend. 2003;72:225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Roberts DM, Joers JM. Neuroimaging in human MDMA (Ecstasy) users. Ann NY Acad Sci. 2008a;1139:291–298. doi: 10.1196/annals.1432.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wood J, Dietrich MS, de Frederick BB, Lukas SE, Renshaw PF. Differential effects of -amphetamine on red and blue light-induced photic activation: a novel BOLD fMRI assay of human dopamine function. Synapse. 2008b;62:268–272. doi: 10.1002/syn.20491. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Klugman A, Baldeweg T, Gruzelier JH. Electrophysiological evidence of serotonergic impairment in long-term MDMA (‘ecstasy') users. Am J Psychiatry. 2001;158:1687–1692. doi: 10.1176/appi.ajp.158.10.1687. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fimm B, Willmes K, Thron A, Gouzoulis-Mayfrank E. Cerebral activation in abstinent ecstasy (MDMA) users during a working memory task: a functional magnetic resonance imaging (fMRI) study. Brain Res Cogn Brain Res. 2003;16:479–487. doi: 10.1016/s0926-6410(03)00075-2. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Heekeren K, Henke K, Thron A, Gouzoulis-Mayfrank E. Memory-related hippocampal dysfunction in poly-drug ecstasy (3,4-methylenedioxymethamphetamine) users. Psychopharmacology (Berl) 2005;180:607–611. doi: 10.1007/s00213-004-2002-8. [DOI] [PubMed] [Google Scholar]
- Daumann J, Jr, Fischermann T, Heekeren K, Thron A, Gouzoulis-Mayfrank E. Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:349–355. doi: 10.1016/j.biopsych.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev. 2010;62:233–244. doi: 10.1016/j.brainresrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA (European Monitoring Center for Drugs and Addiction) 2010.
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, et al. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. American Psychiatric Press: Washington, DC; 1997. [Google Scholar]
- Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/−)3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') J Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Ferrucci M, Lazzeri G, Faetti M, Giusiani M, et al. Previous exposure to (+/−)3,4-methylenedioxymethamphetamine produces long-lasting alteration in limbic brain excitability measured by electroencephalogram spectrum analysis, brain metabolism and seizure susceptibility. Neuroscience. 2005;136:43–53. doi: 10.1016/j.neuroscience.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci. 1999;19:5096–5107. doi: 10.1523/JNEUROSCI.19-12-05096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs MH, Slee SJ, Spain WJ. Diversity of gain modulation by noise in neocortical neurons: regulation by the slow afterhyperpolarization conductance. J Neurosci. 2006;26:8787–8799. doi: 10.1523/JNEUROSCI.1792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (‘ecstasy') users: possible relationship to neurotoxic effects. Psychopharmacology (Berl) 2004;173:383–390. doi: 10.1007/s00213-003-1679-4. [DOI] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, Salmon DP. Asymmetry in auditory and spatial attention span in normal elderly genetically at risk for Alzheimer's disease. J Clin Exp Neuropsychol. 2005;27:240–253. doi: 10.1080/13803390490515441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, de Win MM, van der Tweel I, Schilt T, Kahn RS, van den BW, et al. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008a;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, van der Tweel I, Schilt T, Kahn RS, van den BW, et al. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008b;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La GR, Mahoney JJ, III, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Karageorgiou J, Dietrich MS, Charboneau EJ, Woodward ND, Blackford JU, Salomon RM, et al. Prior MDMA (Ecstasy) use is associated with increased basal ganglia-thalamocortical circuit activation during motor task performance in humans: an fMRI study. NeuroImage. 2009;46:817–826. doi: 10.1016/j.neuroimage.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen SN, Spigset O, Slordal L. The dark side of ecstasy: neuropsychiatric symptoms after exposure to 3,4-methylenedioxymethamphetamine. Basic Clin Pharmacol Toxicol. 2008;102:15–24. doi: 10.1111/j.1742-7843.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, Tong J, McCluskey T, Wilkins D, et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: a positron emission tomography/[(11)C]DASB and structural brain imaging study. Brain. 2010;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SP, Gretton A, Macke J, Logothetis NK. Comparison of pattern recognition methods in classifying high-resolution BOLD signals obtained at high magnetic field in monkeys. Magn Reson Imaging. 2008;26:1007–1014. doi: 10.1016/j.mri.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Liester MB, Grob CS, Bravo GL, Walsh RN. Phenomenology and sequelae of 3,4-methylenedioxymethamphetamine use. J Nerv Ment Dis. 1992;180:345–352. doi: 10.1097/00005053-199206000-00001. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. Effects of (+/−)3,4-methylenedioxymethamphetamine (MDMA) on sleep and circadian rhythms. Scientific World J. 2007;7:231–238. doi: 10.1100/tsw.2007.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Sgambati FP, Schwartz AR, Ricaurte GA. Sleep apnea in young abstinent recreational MDMA (‘ecstasy') consumers. Neurology. 2009a;73:2011–2017. doi: 10.1212/WNL.0b013e3181c51a62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (‘Ecstasy') on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, et al. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (‘ecstasy') users: relationship to cognitive performance. Psychopharmacology (Berl) 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wilson MJ, Sgambati FP, Ricaurte GA. Sleep deprivation differentially impairs cognitive performance in abstinent methylenedioxymethamphetamine (‘Ecstasy') users. J Neurosci. 2009b;29:14050–14056. doi: 10.1523/JNEUROSCI.4654-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M, Bosch-Morell F, Johnsen-Soriano S, Barcia J, Almansa I, Asensio S, et al. Oxidative stress in rat retina and hippocampus after chronic MDMA (‘ecstasy') administration. Neurochem Res. 2007;32:1156–1162. doi: 10.1007/s11064-007-9285-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Dougherty DM, Narayana PA, Kramer LA, Renshaw PF. Functional MRI study of working memory in MDMA users. Psychopharmacology (Berl) 2004;177:185–194. doi: 10.1007/s00213-004-1908-5. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau AW, Amar M, Le RN, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- NIH 2008aNCT00353938: Study of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in people with posttraumatic stress disorderAvailable at: http://www.clinicaltrials.gov .
- NIH 2008bNCT00402298: randomized placebo-controlled study of MDMA-assisted psychotherapy in people with PTSD—IsraelAvailable at: http://www.clinicaltrials.gov .
- NSDUH (National Survey on Drug Use and Health) US Substance Abuse and Mental Health Services Administration. 2009. [PubMed]
- Oliveri M, Calvo G. Increased visual cortical excitability in ecstasy users: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatry. 2003;74:1136–1138. doi: 10.1136/jnnp.74.8.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peat MA, Warren PF, Gibb JW. Effects of a single dose of methamphetamine and iprindole on the serotonergic and dopaminergic system of the rat brain. J Pharmacol Exp Ther. 1983;225:126–131. [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V, Liang H, Woodward N, Bauernfeind A, Lee J, Dietrich M, et al. MDMA (ecstasy) use is associated with reduced BOLD signal change during semantic recognition in abstinent human polydrug users: a preliminary fMRI study. J Psychopharmacol. 2009;24:197–201. doi: 10.1177/0269881109103203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci USA. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff FA, et al. The acute and chronic effects of MDMA (‘ecstasy') on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology. 2002;26:387–396. doi: 10.1016/S0893-133X(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Finnegan KT, Irwin I, Langston JW. Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann NY Acad Sci. 1990;600:699–708. doi: 10.1111/j.1749-6632.1990.tb16919.x. [DOI] [PubMed] [Google Scholar]
- Roberts GM, Nestor L, Garavan H. Learning and memory deficits in ecstasy users and their neural correlates during a face-learning task. Brain Res. 2009;1292:71–81. doi: 10.1016/j.brainres.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–133. doi: 10.1016/s0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Liu X, Chen C. Frequency-dependent modulation of retinogeniculate transmission by serotonin. J Neurosci. 2004;24:10950–10962. doi: 10.1523/JNEUROSCI.3749-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O'Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy') users. Br J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20:22–33. [PubMed] [Google Scholar]
- Taffe MA, Davis SA, Yuan J, Schroeder R, Hatzidimitriou G, Parsons LH, et al. Cognitive performance of MDMA-treated rhesus monkeys: sensitivity to serotonergic challenge. Neuropsychopharmacology. 2002;27:993–1005. doi: 10.1016/S0893-133X(02)00380-9. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH. MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav. 2003;76:141–152. doi: 10.1016/s0091-3057(03)00217-x. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Davis S, Huitron-Resendiz S, Schroeder R, Parsons LH, et al. Functional consequences of repeated (+/−)3,4-methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neuropsychopharmacology. 2001;24:230–239. doi: 10.1016/S0893-133X(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vannini P, Almkvist O, Dierks T, Lehmann C, Wahlund LO. Reduced neuronal efficacy in progressive mild cognitive impairment: a prospective fMRI study on visuospatial processing. Psychiatry Res. 2007;156:43–57. doi: 10.1016/j.pscychresns.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Dersch CM, Rothman RB. Restoration of 3,4-methylenedioxymethamphetamine-induced 5-HT depletion by the administration of -5-hydroxytryptophan. Neuroscience. 2007;148:212–220. doi: 10.1016/j.neuroscience.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 1990;514:276–292. doi: 10.1016/0006-8993(90)91422-d. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Ricaurte GA, Molliver ME. Distinct morphologic classes of serotonergic axons in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylenedioxymethamphetamine. Neuroscience. 1989;28:121–137. doi: 10.1016/0306-4522(89)90237-6. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O'Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am Coll Health. 2006;55:99–104. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann NY Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Sasa M, Takaori S. Serotonin-mediated inhibition from dorsal raphe nucleus of neurons in dorsal lateral geniculate and thalamic reticular nuclei. Brain Res. 1984;290:95–105. doi: 10.1016/0006-8993(84)90739-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.