An apo form of human arginase I which is suitable for soaking experiments has been crystallized.
Keywords: arginase, seeding, alternative reservoirs
Abstract
Arginase (EC 3.5.3.1) is an aminohydrolase that acts on l-arginine to produce urea and ornithine. Two isotypes of the enzyme are found in humans. Type I is predominantly produced in the liver and is a homotrimer of 35 kDa subunits. Human arginase (hArginase) I is seen to be up-regulated in many diseases and is a potential therapeutic target for many diverse indications. Previous reports of crystallization and structure determination of hArginase have always included inhibitors of the enzyme: here, the first case of a true apo crystal form of the enzyme which is suitable for small-molecule soaking is reported. The crystals belonged to space group P212121 and have approximate unit-cell parameters a = 53, b = 67.5, c = 250 Å. The crystals showed slightly anisotropic diffraction to beyond 2.0 Å resolution.
1. Introduction
Arginases are found in organisms as diverse as bacteria and mammals, where they catalyse the reaction arginine + H2O → urea + ornithine (Ash, 2004 ▶). Two isotypes of the enzyme are found in humans. Arginase I is predominately found in the cytoplasm of liver cells, where it catalyzes the final step of urea biogenesis (Ash, 2004 ▶). This function is critically important, as the average adult human excretes ∼10 kg of urea each year (Kanyo et al., 1996 ▶). Arginase I is also found in macrophages, where it is reciprocally transcriptionally regulated with NO synthase and is implicated in macrophage involvement in wound healing (Munder et al., 2006 ▶). The second isotype (arginase II or kidney arginase) is found in the mitochondria of many organs, including brain, spinal cord, kidney, breast etc. (Morris, 2009 ▶). Although the primary function of arginase I appears to be catalysis of the fifth and final step in the urea cycle, the enzyme is the most up-regulated protein observed in the spinal fluid of a mouse model of multiple sclerosis, as well as being up-regulated in asthmatic lung tissue (Maarsingh et al., 2009 ▶). The enzyme has also been shown to promote tumour growth in lung and colorectal cancers (Zea et al., 2005 ▶).
Human arginase I (hArginase I) is a soluble enzyme which is a homotrimer of 322 residues with a binuclear manganese cluster in each of the three independent active sites (Di Costanzo et al., 2005 ▶; Kanyo et al., 1996 ▶). The first X-ray structure of arginase I was of the rat liver enzyme and revealed the bi-atomic manganese centre which is the hallmark of this enzyme (Kanyo et al., 1996 ▶). Previous work has provided high-resolution crystal structures of hArginase I with the tight-binding substrate analogues 6-boronohexanoic acid (ABH) and S-(2-boronoethyl)-l-cysteine (BEC), along with various other molecules (Shishova et al., 2009 ▶; Ilies et al., 2010 ▶; Di Costanzo et al., 2005 ▶, 2007 ▶, 2010 ▶; Baggio et al., 1997 ▶). There has also been a report of an unliganded hArginase I structure (PDB code 2pha): in this instance, the protein was crystallized in the presence of a small molecule which was not seen in the subsequent structure (Di Costanzo et al., 2007 ▶). All reports of hArginase I structures published to date found the enzyme in the same trigonal crystal form, which is hemihedrally twinned much of the time.
We were interested in developing a crystallization system for hArginase I which would yield protein crystals that were suitable for soaking in small molecules: fragments and molecules derived from fragments. The ideal system would be at room temperature and would accommodate the compound being added directly to a crystal-containing droplet. Crystal forms which contained a weak binding inhibitor would be less preferred, but it has been shown that these may work as fragment-soaking systems, albeit with more manual manipulation of the crystal (Newman et al., 2009 ▶).
2. Materials and methods
2.1. Cloning and overproduction
The DNA coding for the 332 residues of hArginase I was inserted into the vector pET24a to give pET24_ArgI. Escherichia coli BL21-AI (Invitrogen, California, USA) cells were transformed with pET24_ArgI and single colonies were picked and tested for overproduction of hArginase I. All colonies tested positive and a starter culture was grown in LB medium to seed a 10 l fermenter with fortified LB (FLB) medium supplemented with 30 mg l−1 kanamycin at 310 K until a cell density (OD600) of 2.6 was obtained. At this point, protein production was induced by addition of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and 0.2% arabinose. After induction, the culture was maintained at 310 K for a total of 4 h (final OD600 of 11.1), after which the cells were harvested by centrifugation and frozen in five tubes containing about 22 g each (108 g in total).
2.2. Purification
Many elements of the purification were derived from the literature (Ikemoto et al., 1990 ▶). Briefly, the cell pellet was resuspended in 10 mM Tris–HCl pH 8, 150 mM NaCl, 1 mM EDTA (10 ml buffer per gram of pellet), which was followed by the addition of MnCl2 to 5 mM and β-mercaptoethanol to 2 mM. To facilitate cell lysis and removal of DNA, 0.25 mg ml−1 lysozyme (Sigma L6876), 2.5 units ml−1 Benzonase Nuclease (Novagen 71205; 250 units µl−1) and 1 mM PMSF (Sigma P7626) were added and the cells were incubated on ice for 15 min.
The cells were lysed by three passages through an Emulsiflex-C5 homogenizer operating at 103 MPa (Avestin, Canada) at 277 K. The lysate was clarified by centrifugation (22 500 rev min−1 for 45 min at 277 K). The supernatant was heated to 333 K for 30 min, cooled on ice and centrifuged at 22 500 rev min−1 for 15 min.
The supernatant containing hArginase I was purified by ion-exchange chromatography on a 5 ml HiTrapSP column (GE Healthcare) equilibrated with low ionic strength buffer (10 mM Tris–HCl pH 7.5, 20 mM NaCl, 1 mM MnCl2, 2 mM β-mercaptoethanol). hArginase I was eluted with a gradient of 0.02–1.0 M NaCl. To polish, the eluate was further purified on a HiLoad 26/60 Superdex 200 gel-filtration column (GE Healthcare) pre-equilibrated with 50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM MnCl2 (HBS + MnCl2).
An average yield was 95 mg purified protein per litre of culture and this was concentrated from 33 to 65 mg ml−1. The protein was portioned into 100 µl aliquots, flash-frozen in liquid nitrogen and stored at 193 K.
2.3. Crystallization
Initial crystallization trials were set up with hArginase I at 6.4 mg ml−1 in HBS + MnCl2 supplemented with 4 mM BEC (K d = 270 nM) or ABH (K d = 5 nM) (Di Costanzo et al., 2005 ▶) and 20 mM MnCl2. Initial trials were performed with three 96-condition screens: the CP-CUSTOM IV screen (Axygen), PEG/Ion HT (Hampton Research) and the CSIRO in-house C3_4 screen. The experiments consisted of 150 nl protein solution mixed with 150 nl crystallant equilibrated against 50 µl crystallant at 293 or 281 K in SD-2 sitting-drop plates (IDEX Corp., California, USA). After two weeks, crystals appeared in condition E12 of the Axygen screen (23.28% PEG 6000, 0.1 M sodium citrate pH 6.5) for the hArginase I–ABH complex. These crystals were used to create a microseed stock which was used to set up more extensive ‘matrix-seeding’ screening trials (D’Arcy et al., 2007 ▶; Ireton & Stoddard, 2004 ▶), in which the protein was complexed with either ornithine or canavarine. Droplets consisted of 200 nl protein solution plus 180 nl crystallant and 20 nl seed stock.
It was clear that the protein could be concentrated to very high levels and we wondered whether we would see different crystal forms using a more concentrated protein stock. The protein was concentrated to 53 mg ml−1 in HBS + MnCl2 and further ‘matrix-seeding’ screening experiments were set up in the same manner: these screens again focused on mid-weight PEG-rich screens and protein with and without added ornithine. Hits consisting of thin multiple plates were observed in conditions containing 18–22% PEG MME 2000 and either malate–MES–Tris (MMT; Newman, 2004 ▶) buffer pH 7 or bis-tris buffer pH 6.5. These experiments were repeated with a second batch of protein that had been concentrated to 33 mg ml−1. A similar crystal was observed with the new batch of protein in PEG 8000 with MMT pH 5. Additive optimization was attempted with Additive Screen HT (Hampton Research) and the Optisalt Suite (Qiagen) using a base condition of 18% PEG 8000, 10%(v/v) MMT buffer pH 5 and seeding. Although significant crystal-quality improvement was seen with the additive screens, we could not reproduce the crystals grown with additives. A further set of additive screening/seeding experiments was performed with the hArginase I protein alone using an in-house protic ionic liquid (PIL) additive screen with the same PEG 8000/MMT base condition. This additive screen consists of 48 different aqueous solutions of ionic liquids, which were tested at final concentrations of both 0.2 M and 20 mM (a communication describing further details of the PIL additive screen is in preparation). A very promising crystal was seen in a condition containing the additive diethylammonium formate. This crystal dissolved over time and a mass of smaller crystals regrew from the shadow of the original crystal. As this decay and regrowth was consistent, we tried setting up the crystallization droplets against a salt solution (Newman, 2005 ▶). Sodium chloride reservoirs at different concentrations (0.1–2.0 M) were tested as possible reservoir solutions and crystals were obtained in droplets set up against reservoirs containing 0.8 M sodium chloride. Other crystallization trials were performed in which small quantities of DMSO were added to the protein, as this would potentially ease future soaking protocols. Crystallization trials consisting of protein in MnCl2/NaCl/HEPES buffer with 5% DMSO set up against 18% PEG 8000, 10%(v/v) MMT pH 5 with PIL additives over 0.6–0.8 M NaCl (with microseeding) reliably yielded a mass of crystals. Trials were set up in which the protein concentration was stepped down from 33 to 10 mg ml−1 (still with 5% DMSO) and the 10 mg ml−1 protein concentration gave droplets containing fewer, more optically perfect crystals.
The seed stock was created using a 3 mm Teflon sphere in a 1.6 ml Eppendorf tube according to the method described by Luft & DeTitta (1999 ▶). In order to produce a very robust seed stock, we created one batch of cross-linked seeds: in this case, the MMT buffer was omitted from the seed solution as the Tris component of the MMT buffer would swamp the glutaraldehyde cross-linking reaction. We added 2 µl 20% PEG 8000 over a droplet containing crystals. Once it appeared that the crystals were not going to dissolve, we transferred the crystals into 50 µl 20% PEG 8000 and vortexed for 1 min. 1 µl of a 2.5% glutaraldehyde stock (1:10 dilution into 20% PEG 8000 of a commercial 25% glutaraldehyde solution; Sigma 85191) was added to the 50 µl seed stock and mixed. The volume of the seed stock was then diluted to 1 ml with 20% PEG 8000.
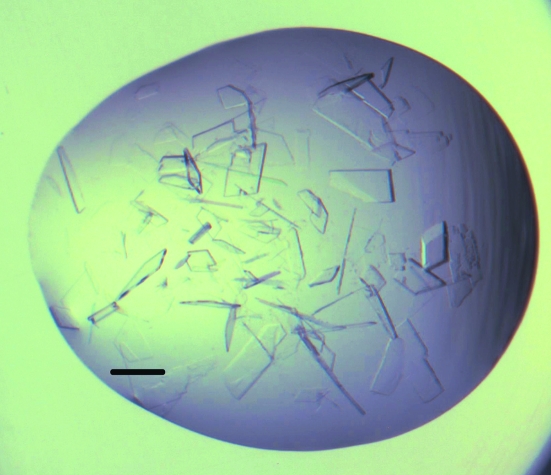
For crystal production, we created a 96-well V-bottomed microplate (Greiner 651101) which contained the ionic solution additive screen pre-mixed with the PEG 8000/MMT base condition in a 1:9 volume ratio. We used a Phoenix dispenser (Art Robbins Industries, California, USA) to add 50 µl 0.65 M NaCl to the reservoirs of 96-well SD-2 crystallization plates and to dispense 250 nl of the protein sample (10 mg ml−1 with 5% DMSO). The plate was then transferred by hand to a Mosquito dispenser (TTP Labtech, UK), where the crystallization droplets were completed by the addition of 225 nl of the pre-mixed additive and base condition and a 25 nl aliquot of microseed stock. The plates were sealed and stored at 293 K. Crystal growth occurred within hours and crystals reached full size within a few days. Cocrystallizations were performed in the same manner, but with the protein solution supplemented with 5% of a DMSO stock of the small molecule of interest at 80–100 mM (see Fig. 1 ▶ and Table 1 ▶).
Figure 1.
Crystals of hArginase I with DMSO and the proprietary fragment grown in PEG 8000, MMT pH 5 with 2-(2-hydroxyethoxy)ethylammonium nitrate as an additive. The protein concentration was 10 mg ml−1. The bar represents 150 µm.
Table 1. Sample information.
| Macromolecule details | |
| Component molecules | hArginase I (EC No. 3.5.3.1) |
| Macromolecular assembly | The molecule is assumed to be a homotrimer of 322 amino acids per monomer |
| Mass (Da) | 34735 (estimated from sequence) |
| Source organism | Homo sapiens (details: human gene) |
| Crystallization and crystal data | |
| Crystallization method | Sitting-drop vapour diffusion |
| Temperature (K) | 293 |
| Apparatus | SD-2 plates |
| Crystal-growth time (d) | 0.5–2 |
| Seeding | Microseeded |
| Crystallization solutions | |
| Macromolecule | 250 nl 10 mg ml−1 hArginase I, 5%(v/v) DMSO, 50 mM HEPES pH 7.4, 150 mM sodium chloride, 1 mM manganese(II) chloride |
| Precipitant | 225 nl 18% polyethylene glycol 8000, 10%(v/v) malate–MES–tris pH 5 |
| Reservoir | 50 µl 0.7 M sodium chloride |
| Crystal data | |
| Matthews coefficient VM (Å3 Da−1) | 2.07 |
| Solvent content (%) | 40.6 |
| Unit-cell data | |
| Crystal system, space group | Orthorhombic, P212121 |
| Unit-cell parameters (Å, °) | a = 53, b = 67.3, c = 245, α = β = γ = 90 |
| No. of molecules in unit cell Z | 3 |
2.4. Data collection
Crystals were harvested directly from the drops and flash-cooled, with no further cryoprotection, directly in the cold nitrogen stream of beamline MX2 of the Australian Synchrotron. Two annealing cycles (Harp et al., 1998 ▶) were used to improve the reflection shape, using a plastic card to block the nitrogen stream for 3–5 s each time. The attenuation of the beam was set to 90% and the detector was set at a distance of 300 mm. 0.5° oscillations were taken and a total of 720 frames were obtained, with each frame given a 1 s total exposure time. The data were processed with XDS (Kabsch, 2010 ▶) to index the reflections; POINTLESS (Collaborative Computational Project, Number 4, 1994 ▶) was then used to determine the initial space group and SCALA (Collaborative Computational Project, Number 4, 1994 ▶) was used to scale and merge the reflections. Although the data clearly extended beyond the 1.87 Å resolution reported, the completeness of the data fell off rapidly, as did many of the merging statistics, so 1.87 Å was considered to be the optimal cutoff point (Table 2 ▶). The data were slightly anisotropic, with the data in the nonpreferred direction extending to 2.02 Å resolution. The estimated B factor from the Wilson plot was 10.8 Å2.
Table 2. Data-collection and structure-solution statistics.
Values in parentheses are for the outer shell.
| Diffraction source | Australian Synchroton, beamline ID3M |
| X-ray beam size (mm) | 0.02 × 0.03 |
| Sampling protocol | Monochromator |
| Wavelength (Å) | 0.9548 |
| Detector | ADSC Quantum 315 |
| Temperature (K) | 100 |
| Resolution range (Å) | 20–1.87 (1.97–1.87) |
| No. of unique reflections | 71762 (9585) |
| No. of observed reflections | 896632 |
| Completeness (%) | 98.8 (92.5) |
| Multiplicity | 12.5 (5.3) |
| 〈I/σ(I)〉 | 23.6 (7.5) |
| Rmerge | 0.087 (0.240) |
| Rp.i.m. | 0.025 |
| Data-processing software | XDS and SCALA |
3. Results and discussion
hArginase I was overproduced in E. coli and purified in two steps to give the final product. As the protein is exceptionally stable, a heat-treatment step was included to remove many of the contaminating E. coli proteins. The protein could be readily concentrated to over 50 mg ml−1 in a buffer consisting of 50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM MnCl2.
Previous work suggested that complexes of hArginase I with ABH and BEC could be crystallized from mid-weight PEG-containing conditions. The three screens used for the initial trials were selected for the preponderance of mid-sized PEGs that they contained. We could reproduce the rod-shaped trigonal crystals of the complexed hArginase I described in the literature (Di Costanzo et al., 2005 ▶), although these crystals dissolved over a couple of weeks. Crystals grown at 281 K dissolved more slowly than those grown at room temperature; we believe that this is a consequence of the slower equilibration of the PEG-based systems at the lower temperature. The initial crystals of the tight inhibitor complex crystals were used to find, via matrix seeding, a different crystal form which grew from weak complexes (ornithine) or uncomplexed protein but required a high concentration (>50 mg ml−1) of protein.
With seeding, we could reliably reproduce these unliganded crystals, which grew in 18–20% PEG 8K with 10%(v/v) MMT buffer pH 5. Generally, the crystals grew as very thin intergrown plates. These thin plates showed a long cell dimension in one direction and adopted a low-symmetry space group, P1 or P21, depending on the crystal. The best diffraction seen from these crystals was to 2.6 Å resolution. These crystals were not optimal: they were very fragile and it was difficult to find single crystals. Furthermore, the low symmetry of the crystals was less than ideal for complete and redundant data collection. After trying a number of different commercial additive screens and not being able to reproduce the crystals, it was found that the in-house protic ionic liquid additive screen reliably gave many crystals that were more three-dimensional and more single than the crystals grown without the PILs, but the problem with crystals redissolving remained (Fig. 2 ▶). Setting up the same droplets over a salt reservoir solved the problem of the crystals redissolving and had the added advantage of allowing robust crystal growth at room temperature. Once a system which used microseeding, alternative reservoirs and additives was in place, it was found that the protein concentration needed to be reduced threefold in order to produce single crystals suitable for manual harvesting.
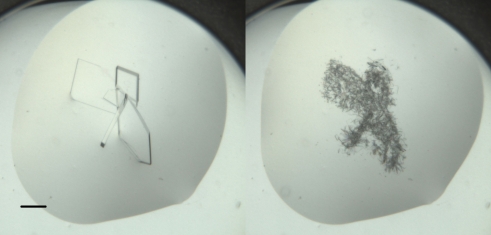
Figure 2.
Crystals of apo hArginase I grown in PEG 8000, MMT pH 5 with 1,1,1,3,3,3-hexafluoro-2-propanol as an additive (Additive Screen HT, well H12). The left-hand image shows crystals that were in the droplet 4 d after setup and the right-hand image shows small crystals that nucleated from the previous crystals; this image was collected 13 d after setup. At 7 d after setup the droplet appeared clear. The bar represents 150 µm.
A seed stock which was cross-linked with glutaraldehyde (and subsequently stored and shipped at room temperature) was tested side by side with a seed stock prepared in a similar manner without the cross-linking step. The cross-linked seeds worked to produce the desired crystal form as reliably as the untreated seeds. This technique may simplify the process of ‘swapping’ seed stocks between different physical locations without the need for expensive shipping (i.e. wet ice or frozen samples).
Crystals grown from this system were flash-cooled without added cryoprotection and were used to collect X-ray diffraction data on the MX2 (microfocus) beamline of the Australian Synchrotron. The crystals belonged to space group P212121, with unit-cell parameters a = 52.5–53.1, b = 67.3, c = 245.2–261.2 Å and with a diffraction limit of 1.8–2.0 Å.
The crystallization system that we have developed was used to create protein crystals suitable for soaking, but could also be used for cocrystallization experiments. These crystallization experiments are a combination of seeding, alternative reservoirs and additive screening, using protein stock supplemented with DMSO to aid both flash-cooling and crystal soaking.
Acknowledgments
The authors acknowledge the use of the CSIRO Collaborative Crystallization Centre. We thank Dr Aaron Oakley for data collection on the low-symmetry crystals and thank the beamline scientists of the MX2 beamline of the Australian Synchrotron for their help with data collection.
References
- Ash, D. E. (2004). J. Nutr. 134, 2760S–2764S. [DOI] [PubMed]
- Baggio, R., Elbaum, D., Kanyo, Z. F., Carroll, P. J., Cavalli, R. C., Ash, D. E. & Christianson, D. W. (1997). J. Am. Chem. Soc. 119, 8107–8108.
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- D’Arcy, A., Villard, F. & Marsh, M. (2007). Acta Cryst. D63, 550–554. [DOI] [PubMed]
- Di Costanzo, L., Ilies, M., Thorn, K. J. & Christianson, D. W. (2010). Arch. Biochem. Biophys. 496, 101–108. [DOI] [PMC free article] [PubMed]
- Di Costanzo, L., Pique, M. E. & Christianson, D. W. (2007). J. Am. Chem. Soc. 129, 6388–6389. [DOI] [PMC free article] [PubMed]
- Di Costanzo, L., Sabio, G., Mora, A., Rodriguez, P. C., Ochoa, A. C., Centeno, F. & Christianson, D. W. (2005). Proc. Natl Acad. Sci. USA, 102, 13058–13063. [DOI] [PMC free article] [PubMed]
- Harp, J. M., Timm, D. E. & Bunick, G. J. (1998). Acta Cryst. D54, 622–628. [DOI] [PubMed]
- Ikemoto, M., Tabata, M., Miyake, T., Kono, T., Mori, M., Totani, M. & Murachi, T. (1990). Biochem. J. 270, 697–703. [DOI] [PMC free article] [PubMed]
- Ilies, M., Di Costanzo, L., North, M. L., Scott, J. A. & Christianson, D. W. (2010). J. Med. Chem. 53, 4266–4276. [DOI] [PMC free article] [PubMed]
- Ireton, G. C. & Stoddard, B. L. (2004). Acta Cryst. D60, 601–605. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kanyo, Z. F., Scolnick, L. R., Ash, D. E. & Christianson, D. W. (1996). Nature (London), 383, 554–557. [DOI] [PubMed]
- Luft, J. R. & DeTitta, G. T. (1999). Acta Cryst. D55, 988–993. [DOI] [PubMed]
- Maarsingh, H., Zaagsma, J. & Meurs, H. (2009). Br. J. Pharmacol. 158, 652–664. [DOI] [PMC free article] [PubMed]
- Morris, S. M. Jr (2009). Br. J. Pharmacol. 157, 922–930. [DOI] [PMC free article] [PubMed]
- Munder, M., Schneider, H., Luckner, C., Giese, T., Langhans, C.-D., Fuentes, J. M., Kropf, P., Mueller, I., Kolb, A., Modolell, M. & Ho, A. D. (2006). Blood, 108, 1627–1634. [DOI] [PubMed]
- Newman, J. (2004). Acta Cryst. D60, 610–612. [DOI] [PubMed]
- Newman, J. (2005). Acta Cryst. D61, 490–493. [DOI] [PubMed]
- Newman, J., Fazio, V. J., Caradoc-Davies, T. T., Branson, K. & Peat, T. S. (2009). J. Biomol. Screen. 14, 1245–1250. [DOI] [PubMed]
- Shishova, E. Y., Di Costanzo, L., Emig, F. A., Ash, D. E. & Christianson, D. W. (2009). Biochemistry, 48, 121–131. [DOI] [PMC free article] [PubMed]
- Zea, A. H., Rodriguez, P. C., Atkins, M. B., Hernandez, C., Signoretti, S., Zabaleta, J., McDermott, D., Quiceno, D., Youmans, A., O’Neill, A., Mier, J. & Ochoa, A. C. (2005). Cancer Res. 65, 3044–3048. [DOI] [PubMed]