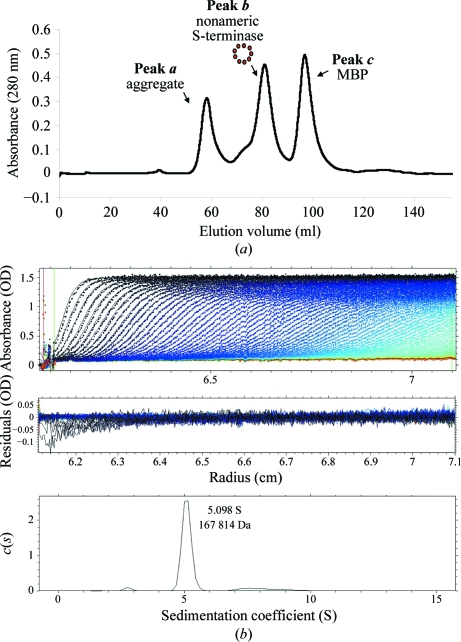
Figure 1.
P22 S-terminase assembles into a nonamer in solution. (a) Size-exclusion chromatogram of overexpressed P22 S-terminase. The Superdex 200 gel-filtration column was calibrated using molecular-mass markers (data not shown). S-terminase elutes as two oligomeric species corresponding to a large aggregate migrating in the void volume (molecular mass >600 kDa; peak a) and a smaller species of ∼200 kDa (peak b). The third peak (peak c) contains free MBP. (b) Sedimentation-velocity profile of P22 S-terminase measured at 15 µM concentration in 0.25 M sodium chloride at 283 K. Top, raw absorbance at 278 nm plotted as a function of the radial position. Data at intervals of 14 min are shown as dots for sedimentation at 30 000 rev min−1. The monophasic sedimentation boundary suggests that S-terminase exists as a monodisperse single species in solution. Middle, the residuals between the fitted curve and the raw data plotted as a function of radius. Bottom, the diffusion-free sedimentation-coefficient distribution c(s) derived from sedimentation velocity of P22 S-terminase corresponds to an estimated molecular mass of ∼167.8 kDa. Given the size of monomeric S-terminase (∼18.5 kDa), this molecular mass is consistent with a nonamer.