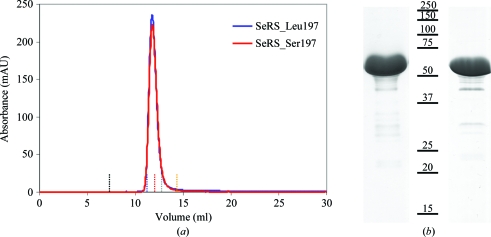
Figure 1.
Purification of C. albicans SerRS isoforms. (a) Analytical gel-filtration chromatography profile of the recombinant proteins showing that they both behave as dimers in solution with an apparent molecular weight of 116 kDa; vertical dotted lines highlight the elution volumes of the molecular-weight markers shown in Table 1 ▶ (black, blue dextran; blue, aldolase; red, albumin; grey, ovalbumin; yellow, chymotrypsin). (b) Coomassie Blue-stained SDS–PAGE (12.5%) analysis of recombinant SerRS isoforms (left, SerRS_Leu197; right, SerRS_Ser197). Both samples contained identical minor impurities that did not interfere with the crystallization process.