Figure 9. NG1427 is directly and reversibly oxidized.
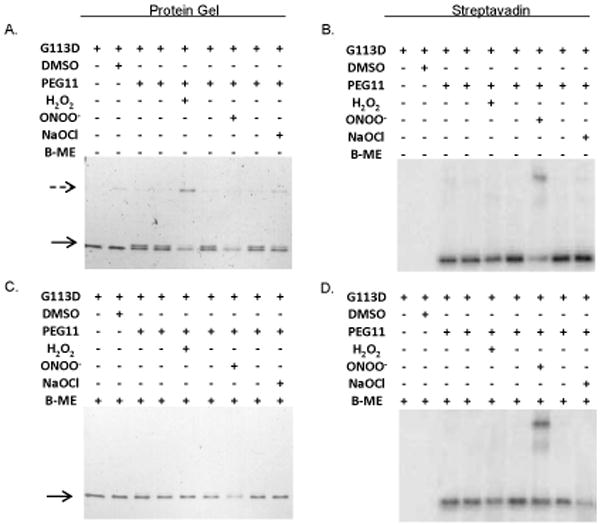
A: Direct visualization of the oxidation of NG1427. 100 nM of NG1427-G113D-HIS was incubated with H2O2, ONOO−, or NaOCl for 15 minutes, then incubated with maleimide-PEG11-biotin. The reactions were then visualized on a non-reducing 4–20% SDS-PAGE gel stained with Coomassie Brilliant Blue. B: Determination of putative NG1427-G113D-HIS dimers free sulfhydryls groups. An equal volume aliquot from (A) was run out on a non-reducing 4–20% SDS-Page gel, transferred to a PVDF membrane, and detected with streptavadin-HRP. C: Examination of reversibility of disulfide-dimers of NG1427-G113D. 100 nM of NG1427-G113D-HIS was incubated with H2O2, ONOO−, or NaOCl for 15 minutes then incubated with maleimide-PEG11-biotin. B-mercaptoethanol (B-ME) was then added to the reactions and were subsequently then visualized on a 4–20% SDS-PAGE gel stained with Coomassie Brilliant Blue. D: Detection of NG1427-G113D dimers following reduction. An equal volume aliquot of B-ME treated samples from (C) were run out on 4–20% SDS-Page gel, transferred to a PVDF membrane, and detected with streptavadin-HRP. The dotted arrow denotes the formation of the higher-molecular weight NG1427-G113D-HIS band, and the lower arrow denotes the mobility shift of NG1427-G113D-HIS following treatment with maleimide-PEG11-biotin.
