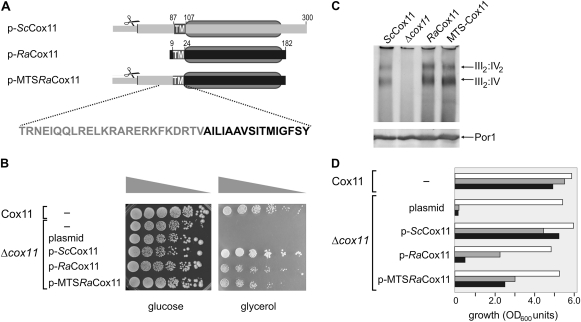
FIG. 3.
Functional complementation of Δcox11 yeast mutants by RaCox11 expressed on a gene in the nucleus. (A) The domain structure of the ScCox11, RaCox11, and MTS-RaCox11 constructs borne by yeast expression plasmids. The MTS-RaCox11 fusion is constructed from residues 1 to 85 of ScCox11 and residues 9–182 from RaCox11. Designated with scissors, an MPP cleavage site is predicted after residue 30 ScCox11. TM denotes the predicted transmembrane segment. The gray oval defines the boundaries of the copper-binding CtaG_Cox11 domain (Pfam 04442) in ScCox11 (residues 105–253) and RaCox11 (residues 25–178). (B) Yeast cells were transformed to express the indicated construct and their growth tested in serial dilution experiments. Equal cell numbers were serially diluted onto medium containing glucose or glycerol as a carbon source and incubated at 25 °C. (C) The transformed yeast strains were grown on medium containing glucose and mitochondria isolated from mid-log phase cultures. Samples of mitochondria (100 μg protein) were analyzed by BN–PAGE, the mitochondrial samples were probed with antisera recognizing the subunit Cox4p to detect cytochrome c oxidase, which migrates as a doublet of bands at ∼1,000 kDa and ∼750 kDa, representing the III2:IV2 and III2:IV supercomplexes formed between cytochrome c oxidase (IV) and cytochrome bc1 reductase (III) as previously described (Schägger and Pfeiffer 2000). Duplicate samples of mitochondria were analyzed by SDS–PAGE and immunoblotting with an antiserum recognizing the outer membrane protein Por1, as a control for the amount of mitochondrial membrane proteins in each of the samples. (D) Transformed yeast cells were grown in liquid media until mid-log phase. The cell numbers measured at 20 h of culture are shown from cultures (white bars) containing glucose as a carbon source, (gray bars) containing glycerol as a carbon source, or (black bars) containing glycerol as a carbon source and supplemented with 50 μM BCDS. The data are representative of five independent experiments.