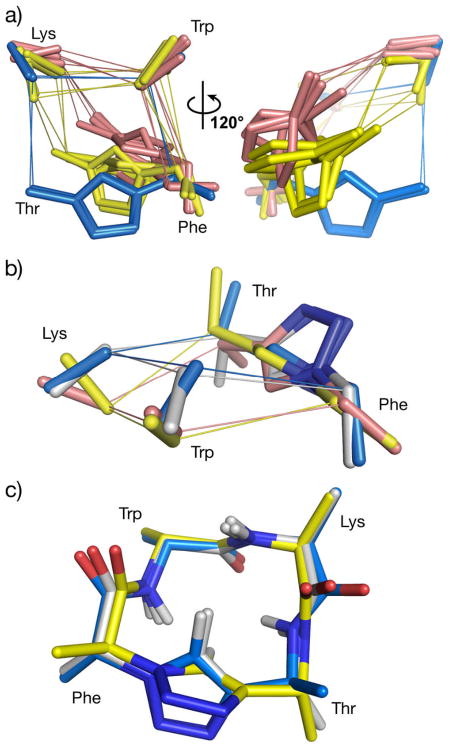
Figure 3.
Pairwise fittings of pseudo-tetrapeptides correlating structure with bioactivity. a) All 16 compounds were overlayed onto ent-7g (highest affinity hSSTR4 binder) using pairfits of the Trp, Lys, and Phe Cβ atoms and the Phe Cα atom. Compounds with an RMSD higher than 0.7 Å are not shown; compounds ent-7g and ent-7d (blue) bind multiple receptors including hSSTR4; compounds ent-7f, 7e, ent-7a, and 7b (gold) are specific for hSSTR4; compounds 7d, 7g, ent-7c, and ent-7h (pink) did not bind or bound with low affinity. Sticks are shown for the triazole atoms and the Cα and Cβ atoms of Phe, Trp, and Lys. b) Overlay of the 16 compounds onto ent-7f (most selective hSSTR4 binder) (pink). An RMSD cutoff of 0.55 Å left compounds 7b (gold), 7e (blue), and ent-7a (white), which represent the hSSTR4-selective peptides. c) Overlay of the 16 compounds using pairfits of the Cα atoms from all four residues and Trp and Lys Cβ atoms onto 7h (most selective hSSTR1 binder) (gold). An RMSD cutoff of 0.3 Å left only molecules ent-7g (blue) and ent-7d (white), which correspond to the only other high affinity, albeit less selective, binders of hSSTR1.