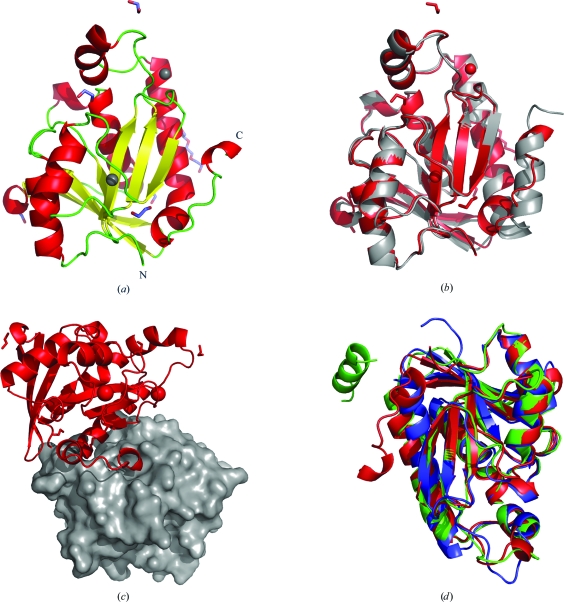
Figure 1.
(a) Ribbon diagram of the secondary structure of F. tularensis peptidyl-tRNA hydrolase (FtPth), with N- and C-termini labeled. The structure comprises a monomer with a single α/β globular domain built around a twisted β-sheet. The coloring scheme for the secondary-structure elements is as follows: α-helices are colored red, β-strands yellow and loops green. Chloride ions are represented by gray spheres, while PEG and ethylene glycol molecules are shown as sticks colored by atom type. (b) Superposition with the published E. coli structure (PDB entry 2pth). This figure was generated using PyMOL (DeLano, 2002 ▶). (c) Crystal-packing diagram for the F. tularensis Pth apo structure. The C-terminus of each molecule (red ribbon) resides in the active site of a neighbouring symmetry mate (gray surface). (d) Superposition of the F. tularensis (colored red), E. coli (colored green; PDB entry 3ofv; R. Lam, T. E. McGrath, V. Romanov, S. A. Gothe, S. R. Peddi, E. Razumova, R. S. Lipman, A. A. Branstrom & N. Y. Chirgadze, unpublished work) and M. tuberculosis (colored blue; PDB entry 2z2i; Selvaraj et al., 2007 ▶) Pth crystal structures. The core secondary-structure elements and protein fold are conserved in all species. As shown by the superposition, the highly mobile C-terminal helix and adjacent hinge region represent opportunities for designing new constructs (for the identification of feasible crystal forms).