Abstract
Background
Multimorbidity is common in primary care populations. Within cardiovascular disease, important differences in disease prevalence and risk factor management by ethnicity are recognised.
Aim
To examine the population burden of cardiovascular multimorbidity and the management of modifiable risk factors by ethnicity.
Design and setting
Cross-sectional study of general practices (148/151) in the east London primary care trusts of Tower Hamlets, City and Hackney, and Newham, with a total population size of 843 720.
Method
Using MIQUEST, patient data were extracted from five cardiovascular registers. Logistic regression analysis was used to examine the risk of being multimorbid by ethnic group, and the control of risk factors by ethnicity and burden of cardiovascular multimorbidity.
Results
The crude prevalence of cardiovascular multimorbidity among patients with at least one cardiovascular condition was 34%. People of non-white ethnicity are more likely to be multimorbid than groups of white ethnicity, with adjusted odds ratios of 2.04 (95% confidence interval [CI] = 1.94 to 2.15) for South Asians and 1.23 (95% CI = 1.18 to 1.29) for groups of black ethnicity. Achievement of targets for blood pressure, cholesterol, and glycated haemoglobin (HbA1c) was higher for patients who were multimorbid than unimorbid. For cholesterol and blood pressure, South Asian patients achieved better control than those of white and black ethnicity. For HbA1c levels, patients of white ethnicity had an advantage over other groups as the morbidity burden increased.
Conclusion
The burden of multiple disease varies by ethnicity. Risk factor management improves with increasing levels of cardiovascular multimorbidity, but clinically important differences by ethnicity remain and contribute to health inequalities.
Keywords: cardiovascular diseases, comorbidity, ethnicity, primary care
How this fits in
Multimorbidity is common in primary care populations. This study illustrates the epidemiology of cardiovascular multimorbidity, showing the high prevalence in South Asians and groups of black ethnicity compared with white populations. Blood pressure and cholesterol are best controlled in the South Asian population. In contrast to other studies, this research shows that overall cardiovascular risk factor management improves with increasing levels of multimorbidity.
INTRODUCTION
Multimorbidity, defined as the simultaneous presence of multiple health conditions, is common in primary care populations, especially for patients over the age of 65 years.1,2 The terms comorbidity and multimorbidity are distinctly defined. Although studies into comorbidity focus on a single index disease of interest and its related conditions, studies into multimorbidity focus on a number of conditions of equal interest without any reference to an index condition.3 The decision to investigate multimorbidity or comorbidity is defined in relation to the research question; studies of multimorbidity are more suited for primary care settings where the focus on a single index condition is less practically relevant.4,5 With the majority of cardiovascular disease managed in primary care, the burden of cardiovascular multimorbidity is of particular concern to GPs.4 Concurrency of multiple cardiovascular conditions has been established as an independent predictor of prognosis in those with established cardiovascular disease.6,7
An evidence-based approach to managing patients with multiple chronic conditions is lacking. In 2005, Fortin et al concluded that:
‘… to date, the number and diversity of articles on comorbidity are both insufficient to provide scientific background for strong evidence-based care of patients affected by multiple concurrent chronic conditions, and … the deficit in research is particularly marked within the primary care setting.8
This deficit in information stems from two primary sources: the exclusion or under-reporting in clinical trials of patients who are multimorbid, and the predominance of single-disease-focused clinical management guidelines.9,10
Ethnicity
Ethnic disparities in the use of health care services are a persistent finding in the UK, in spite of a lack of financial barriers to accessing health care, universal health coverage, and numerous initiatives to reduce disparities.11 People of South Asian descent (Indian, Pakistani, or Bangladeshi origin) who migrate to western countries have some of the highest rates of coronary heart disease (CHD).12,13 In the UK, CHD mortality is up to 40% higher in South Asians but lower in black African and Caribbean groups when compared with groups of white ethnicity. Within each ethnic group, conventional risk factors, such as smoking, hypertension, and total cholesterol, are significant predictors of absolute CHD risk. However, among South Asians, conventional risk factors do not account for the greater prevalence of CHD as compared with Europeans — that is, they do not fully account for between-ethnic-group differences in CHD risk.13
The prevalence of hypertension is considerably higher among black African and Caribbean individuals than among people of white ethnicity,15,16 and the hypertension-associated risk of cardiovascular disease may be accentuated in South Asian groups.17 High-quality management of hypertension is especially important in people of black and South Asian ethnicity, as they are more likely than their white counterparts to have coexisting cardiovascular comorbidities, such as diabetes or chronic kidney disease.18–20
Exactly how the management of risk factors is affected by cardiovascular multimorbidity in different ethnic groups remains to be explored. Although previous studies have focused on comorbidity surrounding a single disease, such as hypertension or diabetes,21,22 no study to date has investigated the overall burden of multiple cardiovascular conditions in a large multi-ethnic population setting.
Study aims
Van den Akker et al reserve the term ‘multimorbidity’ to describe the co-occurrence of two or more chronic conditions.23 This definition was adopted in the present study to examine the population burden of cardiovascular multimorbidity in east London. The study had two aims:
to establish the distribution of cardiovascular multimorbidity between ethnic groups; and
to explore how the management of key physician-modifiable risk factors varies by both ethnicity and level of morbidity.
Specifically, the concurrent presence of CHD, diabetes, heart failure, stroke, and hypertension were investigated. The key risk factors of interest are blood pressure control, total cholesterol control (including statin prescribing), and glycated haemoglobin (HbA1c) control for patients with diabetes.
METHOD
Study sample
Since 1997, the Clinical Effectiveness Group at Queen Mary University of London has supported an annual audit of chronic disease management from 148/151 practices in the three inner east London primary care trusts (PCTs). Routine clinical data entered on practice computers using standard data-entry templates are collected annually using standard MIQUEST software.24
The data presented here were collected during the 15-month period to 31 March 2009. Practice data cover more than 98% of the GP-registered population in the three PCTs. Practices are supported by the Clinical Effectiveness Group to improve identification of chronic disease, use standard data-entry templates at clinical review, and manage conditions based on national guidelines.
The study sample included all adult patients (≥18 years) with diagnostic Read Codes for hypertension, ischaemic heart disease, heart failure, stroke, and diabetes (Appendix 1).
Ethnicity was self-reported by patients during visits to their practices and recorded on the practice computer system using the 16 + 1 ethnicity codes defined in the 2001 census.25 For this study, the ethnic groups were collapsed into four categories:
white (British, Irish, other white);
South Asian (Bangladeshi, Indian, Pakistani, other Asian, mixed Asian);
black (African, Caribbean, black British, mixed black); and
other (any other recorded ethnic group).
Patients whose ethnicity could not be classified from the recorded entry due to non-response or coding error were excluded from the analysis. Patients who identified themselves as mixed origin were grouped with their parent ethnic minority as this was felt to be more biologically relevant than creating a distinct mixed-ethnicity category. Since 2004, a local enhanced service has provided training, data templates, and financial support to promote the recording of ethnicity and language in practices both at registration and during routine consultations.
Multimorbidity and risk factor analysis
The level of multimorbidity was determined by the number of registers on which each patient appeared; if on just one register, they were coded as unimorbid. Patients could have up to five cardiovascular multimorbidities. For the purposes of analysis, patients were collapsed into groups of one morbidity, two morbidities, or three to five morbidities.
The most recent recordings of systolic and diastolic blood pressure, total cholesterol, and HbA1c level during the study period were used for analysis. Clinical targets were based on the National Institute for Health and Clinical Excellence (NICE) guidelines. The targets for each of the risk factors were as follows: a blood pressure of ≤140/90 mmHg, a total cholesterol level of ≤5 mmol/l, and HbA1c ≤7.5%. Each target was coded as a dichotomous categorical variable, with 0 indicating the patient had not reached the target and 1 indicating the target was met. Statin prescribing was coded as 1 if the Read Code was present and 0 if the Read Code was absent.
Statistical analyses
Stata (version 10) was used for both the adjusted and unadjusted univariable and multivariable analyses. First, the crude burden of multimorbidity was examined within the entire study sample and within each ethnic group. Next, the proportion of patients meeting clinical targets by both level of multimorbidity and ethnic group was investigated. For the multivariable analysis, logistic regression was used to examine the odds of being multimorbid by ethnic group, and the likelihood of reaching clinical targets by ethnic group and multimorbidity. All logistic regression analyses were adjusted for age and sex, and clustered by practice to account for intrapractice correlation and shared patient characteristics, such as socioeconomic status. People of white ethnicity were considered as the baseline group for all of the logistic regression analyses.
RESULTS
Population burden of cardiovascular multimorbidity
From a total east London GP-registered population of 843 724 people, a final sample was defined of 99 648 adults recorded as being on at least one of five cardiovascular disease registers in the period 1 January 2008–31 March 2009. Self-reported ethnicity was recorded in 93.6%, blood pressure in 95.8%, and cholesterol in 78.2% of patients in the study sample. HbA1c was recorded in 90.8% of those with diabetes in the study sample. Figure 1 illustrates the relative size and overlap of these five cardiovascular registers in east London.
Figure 1.
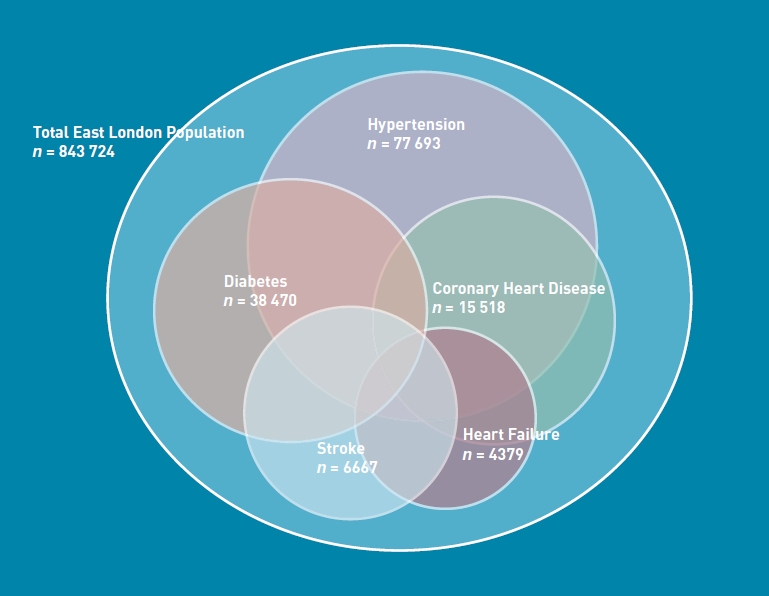
Relative size and overlap of the five cardiovascular disease registers in the total east London population (843 724) in 2009.
Table 1 illustrates the prevalence of cardiovascular multimorbidity in each ethnic group. Although the majority of patients had a single cardiovascular disease, 34% of patients with at least one cardiovascular diagnosis had more than one.
Table 1.
Prevalence of cardiovascular multimorbidity by ethnic group
| Number of morbidities | ||||
|---|---|---|---|---|
| 1 | 2 | 3–5 | ||
| Ethnicity | % (n) | % (n) | % (n) | Total |
| White | 66.4 (26 167) | 25.3 (9963) | 8.3 (3297) | 100 (39 427) |
| South Asian | 59.9 (16 379) | 30.1 (8214) | 10.0 (2742) | 100 (27 335) |
| Black | 69.3 (15 840) | 25.3 (5788) | 5.4 (1231) | 100 (22 859) |
| Other | 67.8 (2440) | 25.7 (926) | 6.5 (235) | 100 (3601) |
Using logistic regression, adjusted for age and sex, and clustered by practice, the likelihood of being multimorbid by ethnicity was then examined (Table 2). It was found that patients of non-white ethnicity were more likely to be multimorbid than those who were white; in particular, South Asian patients were twice as likely to have multiple cardiovascular conditions.
Table 2.
Likelihood of being multimorbid by ethnic group
| Unadjusted odds ratios | Adjusted odds ratiosa | ||
|---|---|---|---|
| Ethnicity | Count | (95% CI) | (95% CI) |
| White | 39 427 | 1.00 | 1.00 |
| South Asian | 27 335 | 1.32 (1.28 to 1.36) | 2.04 (1.94 to 2.15) |
| Black | 22 859 | 0.87 (0.84 to 0.91) | 1.23 (1.18 to 1.29) |
| Other | 3601 | 0.94 (0.87 to 1.01) | 1.22 (1.11 to 1.33) |
Adjusted by age and sex and clustered by general practice.
Management of key risk factors
The management of blood pressure, total cholesterol, and HbA1c within the study sample was examined. As shown in Figure 2, the proportion of patients reaching defined clinical targets was higher in those patients with multimorbidity than those who were unimorbid across all risk factors. The proportion of patients with blood pressure ≤140/90 mmHg rose from 70% to 72%; for HbA1C ≤7.5%, the proportion rose from 50% to 58%. For total cholesterol
Figure 2.
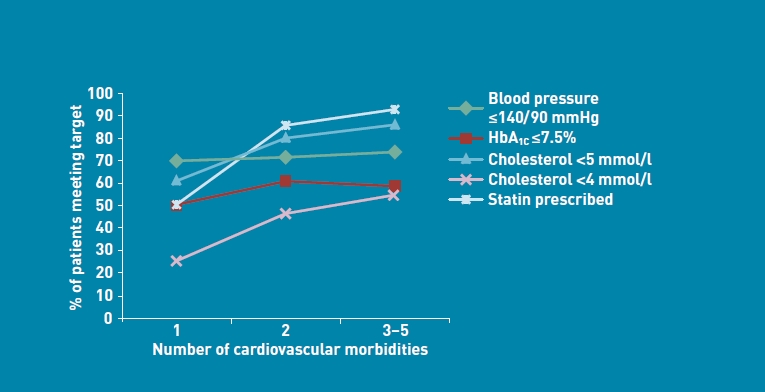
Risk factor control by level of morbidity.
≤5 mmol/l, the proportion rose from 61% to 84%. For the more stringent total cholesterol target of ≤4 mmol, the proportion more than doubled from 25% to 53%. The proportion of patients being prescribed a statin rose from 50% in patients who were unimorbid to 91% in those who were multimorbid.
The multivariable analysis used logistic regression to investigate the likelihood of reaching each clinical target by ethnic group for patients with one condition, two conditions, or three or more conditions. Results are shown in Table 3. For blood pressure, South Asian patients with one or two cardiovascular morbidities were significantly more likely to reach the blood pressure target than patients of white or black ethnicity. No difference was apparent for patients with three or more cardiovascular morbidities. In contrast, patients of black ethnicity were found to be significantly less likely to meet the blood pressure target than patients of white ethnicity at all levels of multimorbidity.
Table 3.
Adjusted odds ratios for key clinical targets, by level of morbidity stratified by ethnicitya
| Adjusted odds ratios (95% CI)b | ||||
|---|---|---|---|---|
| n | 1 morbidity | 2 morbidities | 3–5 morbidities | |
| Reached blood pressure of ≤140/90 mmHg | ||||
| White | 37 942 | 1.00 | 1.00 | 1.00 |
| South Asian | 26 560 | 1.56 (1.43 to 1.71) | 1.27 (1.12 to 1.45) | 1.02 (0.86 to 1.21) |
| Black | 21 712 | 0.75 (0.70 to 0.80) | 0.75 (0.68 to 0.81) | 0.63 (0.53 to 0.75) |
| Other | 3444 | 1.09 (0.98 to 1.22) | 0.99 (0.80 to 1.21) | 0.84 (0.63 to 1.11) |
| Reached cholesterol target of ≤4 mmol/l | ||||
| White | 29 620 | 1.00 | 1.00 | 1.00 |
| South Asian | 23 883 | 2.00 (1.81 to 2.22) | 1.65 (1.49 to 1.83) | 1.60 (1.49 to 1.83) |
| Black | 17 259 | 0.96 (0.90 to 1.03) | 0.96 (0.88 to 1.05) | 0.83 (0.71 to 0.97) |
| Other | 2803 | 1.12 (0.97 to 1.30) | 1.14 (0.96 to 1.36) | 0.91 (0.68 to 1.22) |
| Reached HbA1c target of ≤7.5%c | ||||
| White | 9998 | 1.00 | 1.00 | 1.00 |
| South Asian | 14 988 | 1.01 (0.91 to 1.13) | 0.81 (0.72 to 0.91) | 0.69 (0.60 to 0.79) |
| Black | 7558 | 0.91 (0.81 to 1.04) | 0.91 (0.84 to 0.99) | 0.79 (0.67 to 0.93) |
| Other | 1316 | 1.04 (0.87 to 1.24) | 1.03 (0.86 to 1.24) | 0.81 (0.58 to 1.13) |
Excludes patients without valid indicators.
Adjusted by age and sex, and clustered by general practice.
Analysis restricted to patients with diabetes as one of themorbidities. HbA1c = glycated haemoglobin.
For total serum cholesterol, South Asian patients were consistently more likely to reach the target of ≤4 mmol/l than patients of white ethnicity at all levels of morbidity. No difference was found between those of white and black ethnicity, except among those with three to five conditions, where those of black ethnicity were less likely to meet the cholesterol target.
Patterns in statin prescribing mirrored those for control of total cholesterol. South Asian patients were significantly more likely to be prescribed a statin than those of white ethnicity at every level of morbidity. Although no significant differences were found between patients of black and white ethnicity for cholesterol control, those of black ethnicity were significantly less likely to be prescribed a statin at all levels of morbidity.
For HbA1c control, no differences were found between ethnic groups for unimorbid patients. Among those with two morbidities, both South Asians and patients of black ethnicity were found to have higher levels of HbA1c than white patients. This disparity was increased for patients with
three to five morbidities.
DISCUSSION
Summary
The study was set in the three inner east London PCTs of Newham, Tower Hamlets, and City and Hackney, with a combined GP-registered population of 843 724 in mid-2009. In the 2001 UK census, 51.3% of the population in these three PCTs were of non-white ethnic origin compared with 7.9% for the UK.26 In Hackney, 25.4% were described as black African or black Caribbean, in Newham 21.0% were Indian or Bangladeshi, and in Tower Hamlets 33.3% were Bangladeshi. The populations of these PCTs are among the eight most socially deprived localities in Britain.27
In contrast to other studies that emphasise the relationship between index conditions and secondary morbidities, this study examined the burden of cardiovascular morbidity within a socially deprived and multiethnic population in east London. It shows that the burden of multiple diseases varies by ethnicity, with South Asian groups having the highest rates, and groups of black ethnicity also being significantly more multimorbid than their white counterparts. Overall risk factor management improves with increasing multimorbidity, but clinically important differences by ethnicity remain and can contribute to health inequalities.
Strengths and limitations
This study accessed a database covering a large multi-ethnic population from 98% of practices in the three PCTs of inner east London with uniformly high rates of self-reported ethnicity.20 Related work on this project has shown that when self-reported ethnicity recording is sufficiently high, it should be used in preference to census-attribution methods. Such methods tend to underestimate young people and minority ethnic groups — particularly in inner-city areas — and are subject to the ecological fallacy (the assumption that practice populations are ethnically similar to the census super output areas).28,29 As self-reported ethnicity was recorded for over 95% of the study sample, it is possible to be confident that the relationships between ethnicity and multimorbidity are not prone to the biases of census-attribution methods.
This study has investigated cardiovascular multimorbidity among all adults; hence the analysis is not confined to older populations where multimorbidity has been established as the norm. The analysis shows that, even after adjusting for age and sex, significant differences in the burden and management of cardiovascular multimorbidity by ethnic group remain.
A limitation of the study is the treatment of each of the cardiovascular diseases as equal in impact, and independent of each other. The lack of a unified approach that captures not only disease prevalence, but also disease severity and shared clinical characteristics, has been established as a recurring issue throughout multimorbidity research.2,30,31
The use of South Asian and black categories may have masked intra-ethnic differences, for example between Indian, Pakistani, and Bangladeshi subgroups, which are recognised as being culturally and epidemiologically distinct.32
Although this study was not able to take into account patient-level indices of social deprivation, which can confound the relationship between ethnicity and disease management, the three east London PCTs in this study are all represented in the top eight localities of greatest deprivation in England.27
Comparison with existing literature
Previous studies on the burden and management of multiple chronic diseases have shown that increasing multimorbidity is associated with increased risk of mortality and further morbidity, polypharmacy, and adverse outcomes.1,6,32 In a study investigating the effect of chronic kidney disease and diabetes on hospitalisation and mortality in patients with heart failure, Ritchie et al found that the presence of diabetes in addition to heart failure and chronic kidney disease significantly increased mortality and hospitalisation, and that increasing morbidity was associated with poorer outcomes.33 This study recruited young, predominately white, and male participants and had no data on ethnicity.
Millet et al examined ethnic disparities in the management of hypertension among individuals with and without cardiovascular comorbidities, and found that the presence of two or more comorbidities was associated with significantly improved blood pressure control in patients who were white patients, but not those of black or South Asian ethnicity. The prevalence of cardiovascular comorbidities was higher among South Asian patients with hypertension than among their white or black counterparts. However, in the Wandsworth cohort there was a small South Asian population (5.6%); this is in contrast to the population in the present study, which included a greater percentage of 27% South Asian patients (27%) and those of black ethnicity (23%).32
Randomised controlled trials have been established as the gold standard of clinical study design and are increasingly used to judge quality of care or calculate pay-for-performance targets.1,34 However, to maximise internal validity, these trials often exclude, or do not report, patients with multimorbidity; hence, guidelines based on these randomised controlled trials may not be suitable for the actual patient population in which multiple concurrent conditions are prevalent.1,9,10,35 For example, Fortin et al found that over 90% of patients that were eligible for clinical trials for hypertension had comorbidities.9 The present study, describing the population burden of multiple cardiovascular diseases, strengthens the case for inclusion of patients with multimorbidity in clinical trials, and highlights the need for disease guidelines recognising the prevalence of multiple diseases.
NICE guidelines do not currently take account of comorbidity.36 The present findings show that even in the absence of multimorbid guidelines, patients with cardiovascular multimorbidity have better control of key risk factors, though ethnic disparities persist and require innovative strategies to reduce their impact.
Implications for practice and research
This study shows that the burden of cardiovascular multimorbidity falls disproportionately on patients from minority ethnic groups, and on South Asians in particular. It has also found that the proportion of patients meeting recommended clinical targets typically increases with higher levels of cardiovascular multimorbidity. It is encouraging to find that, despite their increased burden of cardiovascular morbidity, risk factor management for South Asians is generally better, in comparison with other groups, even at higher levels of multimorbidity.
Studies such as this are only possible where there are high rates of ethnicity recording in the general practice record. In areas with large populations from black and minority ethnic groups, commissioning organisations should be encouraged to support practice-level ethnicity and language recording in order to develop accurate estimates of disease prevalence and local population needs. This will allow accurate service evaluation, and support provider initiatives in response to local findings.
Acknowledgments
We thank the staff at the Clinical Effectiveness Group for collating the practice data. Thanks also to the Tower Hamlets Primary Care Trust ethnicity working group, K Boomla, M Caulfield, A Livingston, andMFalshaw, for developing a local enhanced service to promote ethnicity recording.
Appendix 1.
List of Read Codes used in data extraction
| Variable | Read Code |
|---|---|
| Ischaemic heart disease | G3% |
| Diabetes | C10% |
| Hypertension | G2% |
| Stroke | G61, G610, G611, G612, G613, G614, G615, G616, G618, G61X0, G61z, G63y0%, G63y1%, G64%, G66%, G6760%, G6W%, G6X%, G65, G650 to G654, G656 to G65zz, F4236 |
| Heart failure | G58%, G1yz1, 662f to 662i |
| Systolic blood pressure | 2469 |
| Diastolic blood pressure | 246A |
| Total cholesterol | 44P |
| Body mass index | 22K |
| HbA1c level | 44TB, 42W%, 42c%, 44TC |
| Statin prescribed | 8B6A, 8252BRIDL (atorvastatin), 4285EGTON (fluvastatin), 5300EMIS (pravastatin), 6171EMIS (rosuvastatin), 235 (simvastatin), 771 (ezetimibe), 6080BRIDL (bezafibrate), 4946EMIS (ciprofibrate), 15070NEMIS (fenofibrate), 15250NEMIS (gemfibrozil) |
HbA1c = glycated haemoglobin.
Funding
This work was supported by the Health Foundation. All authors are independent fromthe body.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Starfield B. Threads and yarns: weaving the tapestry of comorbidity. Ann Fam Med. 2006;4(2):101–103. doi: 10.1370/afm.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortin M, Bravo G, Hudon C, et al. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223–228. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayliss EA, Ellis JL, Steiner JF. Barriers to self-management and quality-of-life outcomes in seniors with multimorbiditiesy. Ann Fam Med. 2007;5(5):395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valderas JM, Starfield B, Sibbald B, et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schellevis FG, Vandervelden J, Vandelisdonk E, et al. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46(5):469–473. doi: 10.1016/0895-4356(93)90024-u. [DOI] [PubMed] [Google Scholar]
- 6.Glynn LG, Buckley B, Reddan D, et al. Multimorbidity and risk among patients with established cardiovascular disease: a cohort study. Br J Gen Pract. 2008;58(552):488–494. doi: 10.3399/bjgp08X319459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn LG. Multimorbidity: another key issue for cardiovascular medicine. Lancet. 2009;374(9699):1421–1422. doi: 10.1016/S0140-6736(09)61863-8. [DOI] [PubMed] [Google Scholar]
- 8.Fortin M, Lapointe L, Hudon C, Vanasse A. Multimorbidity is common to family practice: is it commonly researched? Can Fam Physician. 2005;51:244–245. [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin M, Dionne J, Pinbo GV, et al. Randomized controlled trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4(2):104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health. London: Department of Health; 2008. Health inequalities: progress and next steps. [Google Scholar]
- 12.McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in South Asians overseas: a review. J Clin Epidemiol. 1989;42(7):597–609. doi: 10.1016/0895-4356(89)90002-4. [DOI] [PubMed] [Google Scholar]
- 13.McKeigue PM, Ferrie JE, Pierpoint T, Marmot MG. Association of early-onset coronary heart disease in South Asian men with glucose intolerance and hyperinsulinemia. Circulation. 1993;87(1):152–161. doi: 10.1161/01.cir.87.1.152. [DOI] [PubMed] [Google Scholar]
- 14.Crawley D, Ng A, Mainous AG 3rd, et al. Impact of pay for performance on quality of chronic disease management by social class group in England. J R Soc Med. 2009;102(3):103–107. doi: 10.1258/jrsm.2009.080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi N, McKeigue PM, Marmot MG. Resting and ambulatory blood pressure differences in Afro-Caribbeans and Europeans. Hypertension. 1993;22(1):90–96. doi: 10.1161/01.hyp.22.1.90. [DOI] [PubMed] [Google Scholar]
- 16.Sproston K, Mindell J. Volume 1: The health of minority ethnic groups. Leeds: The Information Centre; 2006. Health Survey for England 2004. http://www.ic.nhs.uk/webfiles/publications/healthsurvey2004ethnicfull/HealthSurveyforEnglandVol1_210406_PDF.pdf (accessed 17 Mar 2011) [Google Scholar]
- 17.Khattar RS, Swales JD, Senior R, Lahiri A. Racial variation in cardiovascular morbidity and mortality in essential hypertension. Heart. 2000;83(3):267–271. doi: 10.1136/heart.83.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forouhi NG, Merrick D, Goyder E, et al. Diabetes prevalence in England, 2001 — estimates from an epidemiological model. Diabet Med. 2006;23(2):189–197. doi: 10.1111/j.1464-5491.2005.01787.x. [DOI] [PubMed] [Google Scholar]
- 19.Lanting LC, Joung IM, Mackenbach JP, et al. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28(9):2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer G, Hull S, Aitken Z, et al. The effect of ethnicity on the prevalence of diabetes and associated chronic kidney disease. QJM. 2009;102(4):261–269. doi: 10.1093/qjmed/hcn177. [DOI] [PubMed] [Google Scholar]
- 21.Millett C, Bottle A, Ng A, et al. Pay for performance and the quality of diabetes management in individuals with and without co-morbid medical conditions. J R Soc Med. 2009;102(9):369–377. doi: 10.1258/jrsm.2009.090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millett C, Gray J, Wall M, Majeed A. Ethnic disparities in coronary heart disease management and pay for performance in the UK. J Gen Intern Med. 2009;24(1):8–13. doi: 10.1007/s11606-008-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675–679. doi: 10.1016/s0895-4356(00)00358-9. [DOI] [PubMed] [Google Scholar]
- 24.Connecting for Health. MIQUEST. http://www.connectingforhealth.nhs.uk/systemsandservices/data/miquest (accessed 17 Mar 2011). [Google Scholar]
- 25.Office for National Statistics. London: Office for National Statistics; 2008. The census in England and Wales. April 2001. [Google Scholar]
- 26.Office for National Statistics. London: Office for National Statistics; 2009. Population estimates by ethnic group mid-2007. [Google Scholar]
- 27.London Health Observatory. London: London Health Observatory; 2008. Index of Multiple Deprivation. [Google Scholar]
- 28.Hull SA, Rivas C, Bobby J, et al. Hospital data may be more accurate than census data in estimating the ethnic composition of general practice populations. Inform Prim Care. 2009;17(2):67–78. doi: 10.14236/jhi.v17i2.718. [DOI] [PubMed] [Google Scholar]
- 29.MacRae K. Socioeconomic deprivation and health and the ecological fallacy. BMJ. 1994;309(6967):1478–1479. doi: 10.1136/bmj.309.6967.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer SW, Smith SM, Wyke S, et al. Multimorbidity in primary care: developing the research agenda. Fam Pract. 2009;26(2):79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- 31.Kadam UT, Croft PR, North Staffordshire GP Consortium Group. Clinical multimorbidity and physical function in older adults: a record and health status linkage study in general practice. Fam Pract. 2007;24(5):412–419. doi: 10.1093/fampra/cmm049. [DOI] [PubMed] [Google Scholar]
- 32.Millett C, Gray J, Bottle A, Majeed A. Ethnic disparities in blood pressure management in patients with hypertension after the introduction of pay for performance. Ann Fam Med. 2008;6(6):490–496. doi: 10.1370/afm.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: a propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009;134(3):330–335. doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply?’. Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 36.Heyworth ITM, Hazell ML, Linehan MF, Frank TL. How do common chronic conditions affect health-related quality of life? Br J Gen Pract. 2009;59(568):833–838. doi: 10.3399/bjgp09X453990. [DOI] [PMC free article] [PubMed] [Google Scholar]


