Abstract
This study was initiated due to an NIH “Facilities of Research - Spinal Cord Injury” contract to support independent replication of published studies. Transient blockage of the CD11d/CD18 integrin has been reported to reduce secondary neuronal damage as well as to improve functional recovery after spinal cord injury (SCI) in rats. The purpose of this study was to determine whether treatment with an anti-CD11d monoclonal antibody (mAb) would improve motor performance, reduce pain and histopathological damage in animals following clip-compression injury as reported. Adult male Wistar rats (250 g) were anesthetized with isoflurane, and the T12 spinal cord exposed by T10 and T11 dorsal laminectomies followed by a 60 second period of clip compression utilizing a 35 gram clip. Control animals received an isotype-matched irrelevant antibody (1B7) while the treated group received the anti-CD11d mAb (217L; 1.0 mg/kg) systemically. Open-field locomotion and sensory function were assessed and animals were perfusion-fixed at twelve weeks after injury for quantitative histopathological analysis. As compared to 1B7, 217L treated animals showed an overall non-significant trend to better motor recovery. All animals showed chronic mechanical allodynia and anti-CD11d mAb treatment did not significantly prevent its development. Histopathological analysis demonstrated severe injury to gray and white matter after compression with a non-significant trend in anti-CD11d protection compared to control animals for preserved myelin. Although positive effects with the anti-CD11d mAb treatment have been reported after compressive SCI, it is suggested that this potential treatment requires further investigation before clinical trials in spinal cord injured patients are implemented.
Keywords: inflammation, integrin, locomotor, spinal cord injury, rat
Introduction
An important secondary injury mechanism following spinal cord injury (SCI) that is currently a therapeutic target is posttraumatic inflammation (Bethea & Dietrich 2002; Alexander & Popovich, 2009). Acute inflammatory responses following SCI include alterations in the blood-spinal cord barrier, the recruitment and infiltration of circulating inflammatory cells such as neutrophils and monocytes and the subsequent production of proinflammatory cytokines, free radicals and other potentially neurotoxic substances (Chatzipanteli et al., 2002; Loddick & Rothwell, 2002; Nguyen et al., 2007; Alexander & Popovich, 2009). Both experimental and clinical studies have evaluated the inflammatory response to SCI while various mechanistic studies have clarified what cellular adhesion molecules and other processes are activated to recruit these potentially damaging cells to the injured spinal cord (Chatzipanteli et al., 2000, 2002; Fleming et al., 2006).
Various strategies have been utilized to target the acute inflammatory response to SCI, including the use of anti-inflammatory agents as well as blockers of various adhesion molecules (Farooque et al., 1999; Chatzipanteli et al., 2000; Pearse et al., 2003; de Rivero Vaccari et al., 2008; Ankeny & Popovich, 2009; Fleming et al., 2009). It is known that the infiltration and accumulation of activated white cells depends on the upregulation of various leukocyte-endothelial adhesion molecules that lead to the rolling, adhesion and ultimately transmigration of circulating cells (Bevilacqua, 1993; Carlos & Harlan, 1994; Smith, 1993; Hamada et al., 1996). A particular strategy to reduce inflammatory infiltration after SCI has been to prevent the interaction of endothelial cell adhesion molecules with antibodies to the CD11d subunit of the CD11d/CD18 integrin (Grayson et al., 1999; Van der Vieren et al., 1999).
Previous studies have reported that this specific antibody treatment reduces numbers of neutrophils and macrophages in the lesion site after SCI (Mabon et al., 2000; Saville et al., 2002). In a study by Gris and colleagues (2004), transient blockage of the CD11d/CD18 integrin using a monoclonal antibody (mAb) to the CD11d subunit was reported to reduce the infiltration of neutrophils, improve neurological outcomes and reduce pain and histopathological damage following clip compression injury in rats. Given the magnitude and significance of the results using this antibody to the CD11d subunit, and in order to corroborate some of the behavioral findings as a prelude to future clinical trials, antibody treatment to the CD11d subunit was again studied after compressive SCI.
Materials and Methods
Compression Model
Adult male Wistar rats (250 grams, Harlan Laboratories, Frederick, MD, USA) were housed in pairs according to the National Institutes of Health. The Institutional Animal Care and Use Committee of the University of Miami approved all animal procedures. Rats were pre-medicated with diazepam (3.5 mg/kg, i.p.) and atropine (0.05 mg/kg subcutaneously; Sigma Chemical, St. Louis, MO, USA). This sedation procedure facilitates induction of anesthesia by 4% Isoflurane and maintenance by 1.5% (Halothane, used in the Gris et al. (2004) study, is not available in the United States). All surgeries were performed by the same surgeon following the methods outlined by Gris and colleagues (2004). The surgical procedure was directly demonstrated to us by one of Dr. Weaver’s staff members at the University of Western Ontario. Specifically, the T12 spinal cord segment was exposed by T10 and T11 dorsal laminectomies leaving the dura intact. Injury was induced by a 60-second clip compression (Gris et al., 2004). A 35 gram calibrated clip was used at T12 to produce a moderate SCI.
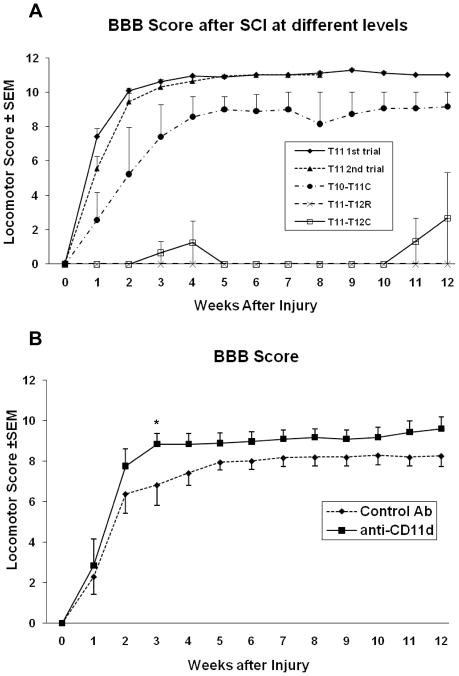
When the parameters for inducing the compression injury were used according to the Gris et al. (2004) study (a single laminectomy at T11, spinal cord level at T12), a less severe locomotor deficit than reported was observed (Figure 1A). The first group of animals (n=12) did not manifest as severe a locomotor deficit, control animals achieving a BBB score of 10 rather than 8 two weeks following a T11 laminectomy. As these animals were slightly heavier in weight than those in the Gris et al. study (275g vs 250g), a second trial was performed with control animals (n=6) weighing 250±5 g. Similar results were obtained up to 8 weeks when the trial was stopped. At this point we considered that the clip might need recalibration and, accordingly, it was sent to Dr. Michael Fehlings’ laboratory. Upon return of the clip, and after communicating with Dr. Weaver, a third trial (n=12) was initiated to compare compression at different spinal cord levels. For this trial the clip was positioned at three different levels (indicated in Figure 1A) after performing double laminectomies because, for BBB testing, the Gris et al. (2004) group had actually performed a double rather than single laminectomy. The scores varied considerably following a double laminectomy at T11 and 12; with the clip placed as rostral or caudal as possible, the resulting deficit implied a complete rather than moderate injury (Figure 1A). It is uncertain whether this difference is due to direct damage to central pattern generator neurons, or to a compromised blood supply after damaging one of the principal radicular arteries from the ventral spinal cord surface (e.g., an equivalent to the artery of Adamkewicz). A compression performed after a double laminectomy yielded poorer BBB scores than when a single laminectomy was done to expose the spinal cord (data not shown). Most likely, the significantly larger laminectomy renders the exposed spinal cord more vulnerable to additional damage and inflammation.
Figure 1.
Open field locomotor scores (BBB). (A) We performed 3 trials to find the equivalent locomotor deficit to that in the original work. In the first two trials a single laminectomy at T11 yielded less severe functional deficits than those reported by Gris et al. (2004). In a third trial, the clip was placed at different levels after performing double laminectomies at T10-T11 or T11-T12 (clip placed as rostral (R) or caudal (C) as possible). (B) Although anti-CD11d mAb treatment led to improved scores during the 12 week observation period, no significant differences between the two groups were demonstrated using two-way repeated measures ANOVA with one repeating factor (time). However, analyzing the data utilizing one-way ANOVA (Gris et al., 2004), there was a significant difference between experimental and control animals at week 3 (*P=0.027).
On the basis of the third trial, and in consultation with Dr. Weaver, it was decided to start the replication study with a double laminectomy at T10-11, with compression at cord level T12. The BBB results now were similar to those published by Gris et al. (2004). For all trials particular attention was paid to avoid “clipping” a piece of muscle, centering the cord to the curvature of the clip, avoiding damage to the dura, carefully timing the 60 seconds of compression, and excluding any cases in which severe bleeding was observed after clip placement. To guarantee correct positioning of the clip, we counted, starting at the dorsal process of T2 (the tallest vertebra in rats), the dorsal processes of the thoracic vertebrae under the skin using a spinal hook.
Post-operative care was provided, and rats were maintained on a heating pad to keep body temperature at 37° for 1 hr. Rats received Bu prinorphine (0.01 mg/kg subcutaneously; Bedford Labs, Bedford, OH) twice a day for 3 days. A bolus of Baytril (20mg/kg) was given immediately after surgery with a maintenance dose of 10 mg/kg subcutaneously twice a day for 3 days.
Rats were randomly assigned to one of two groups when the treatments were administered intravenously via the tail vein (90μl) in three consecutive doses, each 1 mg/kg, at 2, 24 and 48 hours after SCI. The control group received an isotype-matched irrelevant antibody (N=8; 1B7, 1 mg/kg, gift of the ICOS Corporation, Bothel, WA) and a second group received the anti-CD11d mAb (N=6; 2B7 1.0 mg/kg; antibody from the same lot number as the one used in the Gris et al. (2004) study was donated by ICOS Corporation). The experimental antibody was received frozen; when the animals had been injured, the antibody was thawed, divided into aliquots for each animal and stored at 4° until use (to avoid refreezing) for no more than two days. A Senior Research Advisor from Eli Lilly and Co., (acquired ICOS Corporation), confirmed the stability of the antibody. All aspects of the treatment protocol, behavioral and histopathological testing and data analyses were done in a blinded experimental design.
Locomotor function and Mechanical Allodynia Testing
Locomotor recovery of animals was assessed by two independent observers using the Basso, Beattie and Bresnahan (BBB) open field locomotor score (Basso et al., 1995). Animals were tested twice a week up to 12 weeks after injury. BBB scores represent the average of the two testing sessions per week. Inclined plane tests were performed every week starting at week 5 after injury. For this test, rats were placed on a flat board with a rubber surface equivalent to the one used by Gris et al. (2004). The board was tilted with 5° increments from the horizontal plane until the maximal angle at which the rat held its head-up position for 3 seconds was determined.
Rats were also tested every other week for the presence of mechanical allodynia in the dorsal trunk and hindpaws before and 2–12 weeks after SCI as described by Gris et al., (2004). A modified Semmes-Weinstein filament calibrated to generate a force of 15mN was used to test rats for their response to innocuous mechanical stimulation. Ten stimulations with the filament lasting 3 sec each separated by a 5 sec interval comprised each testing session. The number of avoidance responses (paw withdrawal and/or licking, flinching, escape, vocalization, or abnormal aggressive behavior) to 10 stimuli was quantified.
Lesion Assessment
At 12 weeks after injury, all animals were anesthetized and intracardially perfused with cold heparinized saline followed by a 4% paraformaldehyde solution. A 1 cm segment of spinal cord centered on the lesion site was removed from each animal and cryopreserved in 25% buffered sucrose solution. Tissue was then cut transversely in 20 μm serial sections throughout the sample and stained with an antibody to Myelin Basic Protein (MBP) and counterstained with Methyl Green. This staining in our hands was found to be preferable to Solochrome Cyanin and H & E staining for analysis. Data from preserved myelin, penumbra, and cavities were obtained from sections throughout the 1 cm segment (including the epicenter) every 800 microns using a Zeiss Axiovert 200M microscope. Volumes were estimated using the planimetric analysis with Neurolucida software (MBF Bioscience, Inc., Wiliston, VT, USA); 3D reconstructions were made using Neurolucida software (MBF Bioscience, Inc.). Penumbra was defined by tissue with small cell infiltration and the presence of neurons appearing altered in size, number and their normal distribution in the gray matter. Cavities included areas lacking tissue or sparsely filled with necrotic debris. Data for preserved myelin, penumbra, and cavitaty are reported as percentages of the contour (total area of cross section) throughout the analyzed 1 cm segment. This approach was similar to the strategies conducted by Gris and colleagues (2004) where normalized areas of myelin were evaluated at multiple sections rostral and caudal to the lesion epicenter.
Statistical Analysis
Data are expressed as means ± SEM. Statistical analysis of behavioral and histopathological area data between groups was performed using two-way repeated measures ANOVA followed by Student Newman-Keuls test or Tukey’s test, respectively. Histopathology volume percentage data were analyzed using one-way ANOVA. In addition, the BBB data were also analyzed as described in the Gris et al., 2004 paper using one-way ANOVA followed by Fisher’s exact test. Statistical analysis was considered significant at P < 0.05.
Results
Immediately following a moderate compressive injury, all animals displayed very low BBB scores (Figure 1B). During the next several weeks, there was spontaneous recovery which then plateaued around the third to fifth week after injury. After that point, BBB scores stayed relatively unchanged. In control treated rats, the BBB scores stabilized at about five weeks reaching a score of 7.93 ± 0.37. In the mAB-treated rats, BBB scores stabilized 3 weeks after injury at a score of 8.83 ± 0.52. Final BBB scores were 8.25 ± 0.52 and 9.58 ± 0.61 for the control and the anti-CD11d mAb-treated animals, respectively. Thus, although BBB scores in the anti-CD11d mAb-treated group reached a plateau earlier and remained somewhat higher than those of the control, a significant difference between the two groups was not shown (p=0.16). One difference regarding the present analysis and the one done by Gris et al. (2004) is that our data were analyzed using a two-way ANOVA with one repeating factor (Time); however, Gris and colleagues (2004) analyzed their BBB data using one-way ANOVA. When we applied their analysis method to our data we found only week 3 to be significantly different (p=0.027) between groups.
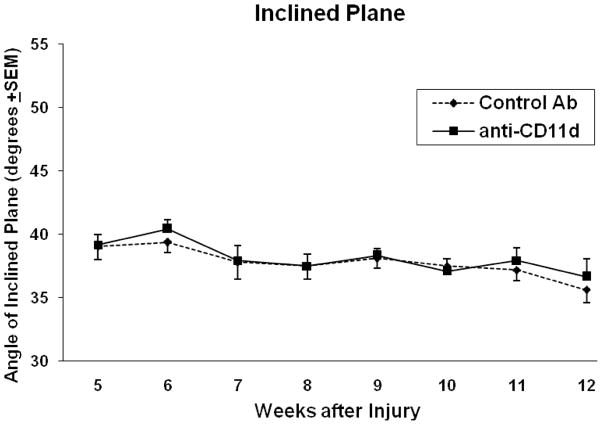
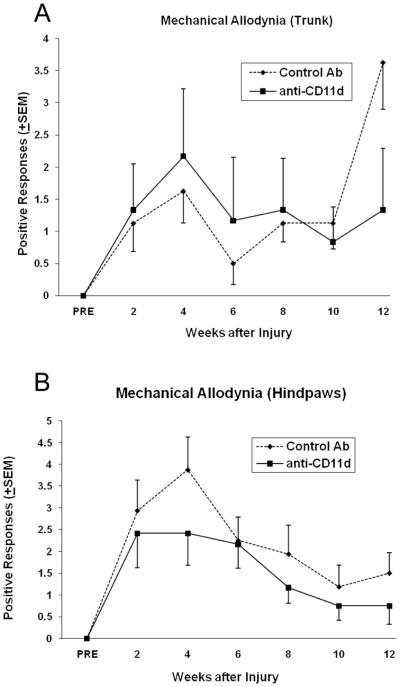
Using the inclined plane test, both the mAB-treated and control rats were able to maintain their position on a tilted platform at about the same angle (Figure 2). Both control and treated animals held position at an average angle of 39° at 5 weeks after injury and approximately 36° at 12 weeks; this decrease over time was not significant for both groups. Evidence for mechanical allodynia was observed in SCI rats between 2–12 weeks after the T12 injury with the greatest difference seen at 12 weeks in the trunk region (Figure 3A). In regards to the dorsal trunk immediately rostral to the lesion, avoidance responses to ten stimuli demonstrated the development of mechanical allodynia. Compared to control treated SCI animals, anti-CD11d mAb treatment decreased the frequency with which these avoidance responses occurred at 12 weeks after injury. The testing of the hindpaws (Figure 3B) showed the highest evidence of mechanical allodynia at 4 weeks and decreased over the 12 weeks for both groups. This decrease was statistically significant (p=0.01) in the control group indicating a decrease in neuropatic pain in the hindpaws opposed to increased neuropathic pain found in the trunk of control animals. Although numbers of avoidance responses tended to be lower in the experimental versus the control group in the hindpaws, no significant differences were observed.
Figure 2.
The inclined plane test determines the ability of the rat to hold its position on an inclined plane with forelimbs and hindlimbs. No significant differences (p>0.05) in the ability of control Ab and anti-CD11d mAb treated rats to maintain their position on a tilted platform was observed up to 12 weeks after injury.
Figure 3.
Following SCI, rats demonstrated evidence of mechanical allodynia in the (A) trunk and (B) hindpaws. Although the number of avoidance responses elicited by mechanical stimulation of the trunk were routinely lower in anti-CD11d-treated rats, no significant differences (p>0.05) between the two groups were observed. When comparing week 4 to week 12 data, trunk and hindpaw mechanical allodynia showed a significant (p<0.01) difference in control-Ab treated animals. There was no significant difference between the anti-CD11d mAb treated rats at 4 and 12 weeks.
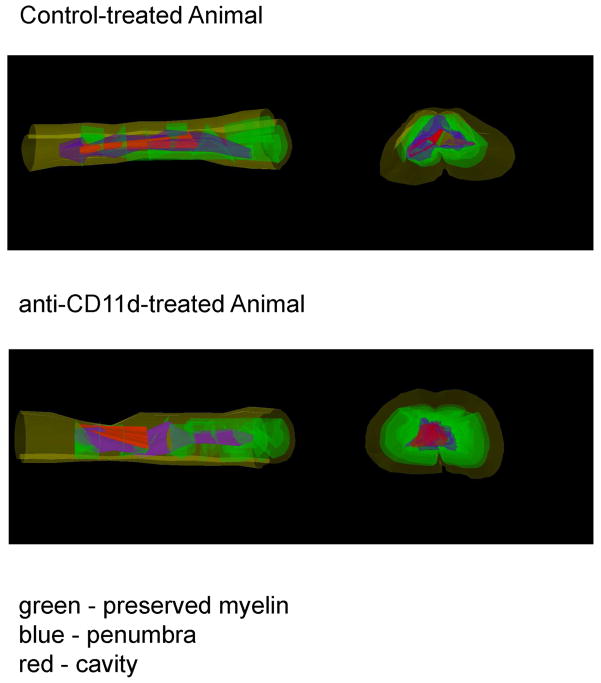
At 12 weeks after injury, animals were perfusion-fixed for histopathological analysis. Areas of preserved myelin, penumbra and cavity were measured and are reported as percentage of the spinal cord contour throughout the injured segment. In some cases 3-D images were created to visualize the extent of damage to the cords in the epicenter and beyond (Figure 4). As shown in Figure 5A, significant histopathological damage was qualitatively seen in the injured cords of control and anti-CD11d-treated rats. Two-way ANOVA was significant for changes in preserved myelin (Figure 5B), penumbra (Figure 5C), and cavity (Figure 5D) across the rostral-caudal extent of the tissue. However, there was only a significant interaction effect of group times level for preserved myelin (Figure 5B; p<0.02). Posthoc analysis indicated a significant difference between groups for −3200 microns and 1600 microns. Because only two levels were significantly different, preserved myelin volumes as a percentage of the total contour did not reach significance (Figure 5E; p=0.07).
Figure 4.
3-D reconstruction of injury patterns in control and anti-CD11d-treated rats. Notice similar patterns of preserved myelin (green), penumbral regions (blue), and cavity formation (red).
Figure 5.
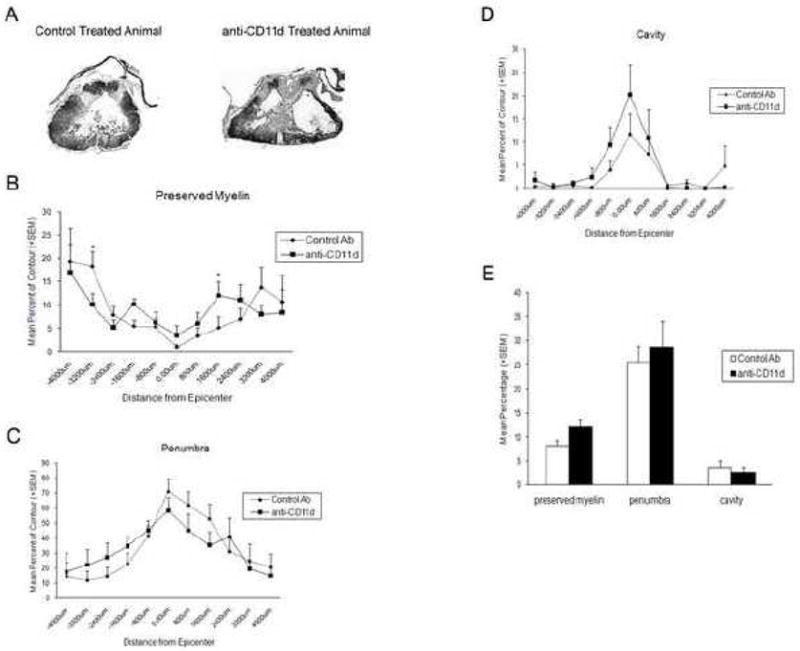
(A) Representative micrographs of rat spinal cord sections stained with Myelin Basic Protein and counterstained with Methyl Green. Well-demarcated cavities were present at the epicenter of the compression injury at 12 weeks after injury with severe damage to gray and white matter. (B–D) Quantitative assessment of percentage of preserved myelin, penumbral areas, and cavity across rostral and caudal regions from the epicenter (0.0 microns) in control and anti-CD11d mAb-treated rats; the negative signs represent the rostral direction. There was a significant interaction between group and level (p<0.05) for preserved myelin. Posthoc analysis was significant between groups at −3200 microns and 1600 microns (*). No significant differences (p>0.05) were observed between the two groups in penumbra or cavity or. (E) Volumetric data represented as percentage of total contour volume also showed no significant differences (p>0.05) between any of the histopathological outcomes.
Discussion
Recently, several publications have advocated the use of anti-inflammatory therapies targeting SCI (Mabon et al., 2000; Saville et al., 2002; Gris et al., 2004; de Rivero Vaccari et al., 2008). Studies have emphasized the importance of posttraumatic inflammatory processes in the pathophysiology of experimental and clinical SCI (Fleming et al., 2006; Alexander & Popovich, 2009). Various strategies including the use of transgenic or knock-out mice as well as specific antibodies to adhesion molecules leading to decreased inflammatory responses have been reported to improve outcome. In addition, studies elucidating the various inflammatory cellular components that appear to represent a secondary injury mechanism have been published (de Rivero Vaccari et al., 2008; Ankeny & Popovich, 2009). Downstream production of pro-inflammatory cytokines and other injurious compounds released by inflammatory cells also have been targeted for therapeutic interventions, again leading to improved outcome (Chatzipanteli et al., 2002). Thus, novel strategies that target newly identified adhesion molecules that participate in the recruitment of inflammatory cells to an injured site are of utmost importance in defining new targets for therapeutic interventions.
Gris and colleagues (2004) found that treatment with the anti-CD11d mAb reduces secondary damage and improves motor and sensory outcomes in a clip compression SCI model. After SCI, intraspinal leukocyte infiltration initially requires leukocyte tethering by selectins on the surface of endothelial cells (Bevilacqua, 1993). This initial response to injury is followed by the interaction of endothelial cell adhesion molecules with integrins on the leukocyte surface (Neish et al., 1995; Shanley et al., 1998) facilitating leukocyte extravasation through the blood-spinal cord barrier. Previous studies utilizing an antibody to the CD11d subunit of the CD11d/CD18 integrin reported decreased numbers of neutrophils and macrophages at the lesion site 2–3 days after SCI (Mabon et al., 2000; Saville et al., 2002). In a study by Gris and colleagues (2004), transient blockage of the CD11d/CD18 integrin was also reported to reduce the devastating sensory and motor consequences of severe SCI and to improve the preservation of white matter tracts.
In a pilot study (Figure 1A), we first attempted to reproduce the behavioral consequences of a 60 second period of clip compression (35 gm clip at the T12 cord level after a single laminectomy), according to Gris et al. (2004). It became apparent that this procedure in our hands produced a less severe locomotor deficit in control animals than that originally published. Minor surgical details are often overlooked; the results from our pilot experiments highlighted the relevance of properly determining the spinal cord level for injury, especially when performing injuries at the lower thoracic level. We recommend using the dorsal process of the second thoracic (T2) vertebra as a reference point for studies using models of SCI, as this is the most reliable anatomic landmark in rats. In consultation with Dr. Weaver we learned that for BBB testing a double rather than a single laminectomy had been performed in her laboratory. The subsequent trials performed to achieve a functional deficit comparable to that in the published work are described in the Materials and Methods section. After a double laminectomy, with compression at spinal cord level T12, the BBB results for control animals were similar to those published by Gris et al. (2004).
The results of the present study showed that early treatment with the antibody to the CD11d subunit of the CD11d/CD18 integrin improved overall BBB function as demonstrated by higher locomotor scores between 2–12 weeks after injury. At 12 weeks post injury, BBB scores in control and anti-CD11d mAb-treated were 8.25 ± 0.52 and 9.58 ± 0.61, respectively. Although these BBB value differences were not statistically significant at most time points after injury, it is important to note that most animals in the treated group achieved weight-supported stepping, a functional difference with clinical relevance (Kwon et al., 2010). Thus, these findings are somewhat consistent with the BBB treatment findings published by Gris and colleagues (2004).
Evidence for the transient blockage of the CD11d/CD18 integrin leading to a reduction in pain was not obtained. In the assessment of mechanical allodynia within the trunk area, anti-CD11d antibody treatment non-significantly reduced the mean number of avoidance responses only at 12 weeks after injury. Also, no obvious effect was seen when pain thresholds were assessed for the hindpaws. Our data demonstrated a slightly lower average number of avoidance responses for the control group than that reported by Gris and colleagues (2004). It is therefore possible that in spite of our effort to replicate the functional deficits in control animals, this model in our hands resulted in a less severe mechanical allodynia. Alternatively, the variability inherent in this test and the myriad of behaviors that are considered positive responses (e.g., flinching, escape, vocalization, or abnormal aggressive behaviors) allow introduction of tester errors due to the subjectivity of the outcome measurement and could explain the differences in results.
In our histopathological studies, we sought to demonstrate that anti-CD11d treatment improved the amount of white matter sparing as compared to control animals. The histopathological assessment was performed similarly to Gris and colleagues (2004). The amount of white matter preservation has previously been shown to be an important factor in determining locomotor outcome as assessed by the BBB test (Basso et al., 1995). Unfortunately, neither significant differences in preserved myelin nor cavity volume were seen between the experimental and control groups. However, the percentage of preserved myelin in the anti-CD11d group was slightly more than in the control group (p=0.07). Significant differences were seen between treatment groups at only sections samples at either 3200 microns rostral or 1600 microns caudal to the lesion epicenter. Thus, although we did observe a slight increase in overall return of locomotor function in anti-CD11d-treated SCI rats, no significant corresponding effect on total myelin preservation was observed in this study.
Despite our considerable endeavour, there is the possibility that the injury severity differed to some degree from the Gris et al. (2004) study and this could have led to different results. An obvious difference between the present study and that of Gris and colleagues (2004) was the type of anesthesia used. In the Gris et al. (2004) manuscript, halothane was utilized whereas, in the present study, isoflurane was used due to the discontinued availability of halothane. It is known that different anesthetics can have significantly different effects on CNS injury outcomes (O’Connor et al., 2003; Yurdakoc et al., 2008). Thus, it is possible that this variable could have influenced the present findings. In addition, the antibody was not handled identically as it was kept at 4 degrees for two days longer than in Dr. Weaver’s laboratory. After thawing the vials received from ICOS, 90μl-aliquots were prepared for injections and then kept at 4°C until used. In Dr. Weaver’s laboratory, the antibodies were received in liquid form and then aliquots were prepared before initial freezing and then thawed immediately before injection. Therefore, we kept the antibody 2 days longer at 4°C to avoid refreezing. We contacted a Senior Research Advisor from Eli Lilly and Co. who confirmed the stability of the antibody; therefore, it is unlikely that this explains why our differences in behavioral recovery did not reach statistical significance. Whereas we set out to do a replication study, some differences arose that were beyond our control. Nevertheless, this was a study that contributes to knowledge about the value of the CD11d subunit of the CD11d/CD18 integrin to protect the injured spinal cord after compression injury.
While earlier research findings reported the beneficial effects of transient blockage of CD11d/CD18 integrin in SCI, it would be relevant if this specific therapy also was tested in a contusive model of SCI. Contusive spinal cord damage due to rapid impact injury is commonly seen in the clinical situation. Whether or not this treatment strategy would improve functional outcome and protect histopathologically in such a model is unknown. In addition, it would be important to conduct a dose response study to verify the optimal therapeutic dose of this agent and determine the therapeutic window of this strategy. It is encouraged that these types of studies be conducted prior to consideration of clinical trials using this therapy in human SCI.
Acknowledgments
We would like to thank Ileana Oropesa, Denise Koivisto, Rosa Abril, Monica Stagg, Ramon German for animal care and behavioral testing; Raisa Puzis for tissue processing; and Jeremy Lytle for expert editorial assistance and word processing. Supported by funds from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Facilities of Research Excellence in Spinal Cord Injury (FORE-SCI) under contract No. N01-NS-3-2352, and The Miami Project to Cure Paralysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158:1112–1121. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Chatzipanteli K, Garcia R, Marcillo AE, Loor KE, Kraydieh S, Dietrich WD. Temporal and segmental distribution of constitutive and inducible nitric oxide syntheses following traumatic spinal cord injury: Effect of aminoguanidine treatment. J Neurotrauma. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K, Yanagawa Y, Marcillo AE, Kraydieh S, Yezierski RP, Dietrich WD. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma. 2000;17:321–332. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak E, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma. 2003;20:533–541. doi: 10.1089/089771503767168465. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooque M, Isaksson J, Olsson Y. Improved recovery after spinal cord trauma in ICAM-1 and P-selectin knockout mice. Neuroreport. 1999;10:131–134. doi: 10.1097/00001756-199901180-00024. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Gonzalez-Lara LE, Foster PJ, Weaver LC. Timing and duration of anti-alpha4beta1 integrin treatment after spinal cord injury: effect on therapeutic efficacy. J Neurosurg Spine. 2009;11:575–587. doi: 10.3171/2009.6.SPINE08915. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129 (Pt. 12):3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Van der Vieren M, Sterbinsky SA, Gallantin WM, Hoffman P, Staunton D, Bochner BS. Alphadbeta2 integrin is a ligand for vascular cell adhesion molecule-1. Int Arch Allergy Immunol. 1999;118:263–264. doi: 10.1159/000024094. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockage of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neuroscience. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y, Fukuzawa K. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996;66:1525–1531. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Tsai E, Beattie MS, Bresnahan J, Magnuson DS, Reier PJ, McTigue DM, Popovich P, Oudega M, Blight AR, Guest J, Weaver L, Fehlings M, Tetzlaff W. A grading system to objectively evaluate the strength of preclinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J Neurotrauma. 2010 May 27; doi: 10.1089/neu.2010.1296. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Cytokines and neurodegeneration. In: Rothwell NJ, Loddick SA, editors. Immune and Inflammatory Responses in the Nervous System. Oxford University Press; Oxford: 2002. pp. 90–105. [Google Scholar]
- Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the Integrin alphaD: a potential new anti-inflammatory treatment. Exp Neurol. 2000;166:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- Neish AS, Read MD, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix-metalloproteinases, reactive oxygen species, and TNF-alpha. J Neurochem. 2007;102:900–912. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Chatzipanteli K, Marcillo AE, Bunge MB, Dietrich WD. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J Neuropathol Exp Neurol. 2003;62:1096–1107. doi: 10.1093/jnen/62.11.1096. [DOI] [PubMed] [Google Scholar]
- Saville LR, Pospisil CH, Mawhinney LA, Bao F, Simedrea FC, Peters AA, O’Connell PJ, Weaver LC, Dekaban GA. A monoclonal antibody CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 2002;156:42–57. doi: 10.1016/j.jneuroim.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Warner RL, Crouch LD, Dietsch GN, Clark DL, O’Brien MM, Gallatin WM, Ward PA. Requirements for an αd in IgG immune complex-induced rat lung injury. J Immunol. 1998;160:1014–1020. [PubMed] [Google Scholar]
- Smith CW. Leukocyte-endothelial cell interactions. Semin Hematol. 1993;30:45–53. [PubMed] [Google Scholar]
- Van der Vieren M, Crowe DT, Hoekstra D, Vazeux R, Hoffman PA, Grayson MH, Bochner BS, Gallatin WM, Staunton DE. The leukocyte integrin αDβ2 binds VCAM-1: evidence for a binding interface between I domain and VCAM-1. J Immunol. 1999;163:1984–1990. [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Yurdakoc A, Gunday I, Memis D. Effects of halothane, isoflurane, and sevoflurane on lipid peroxidation following experimental closed head trauma in rats. Acta Anaesthesiol Scand. 2008;52:658–663. doi: 10.1111/j.1399-6576.2008.01635.x. [DOI] [PubMed] [Google Scholar]