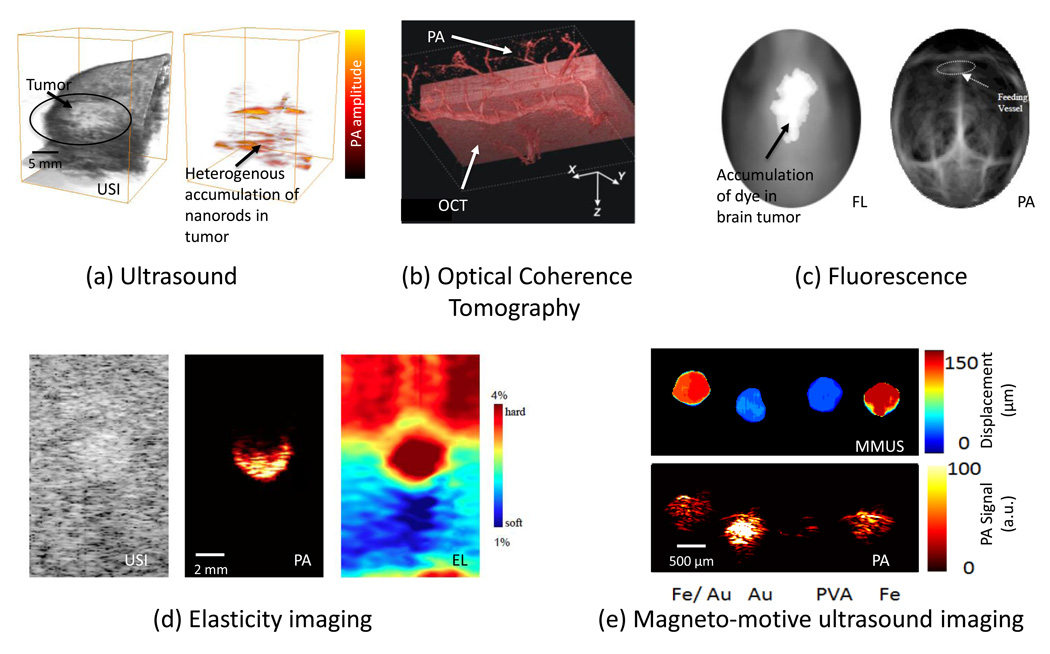
Figure 3.
Various optical- and ultrasound-based imaging techniques can be combined with PAI to provide structural, functional and biomechanical properties of the tissue. (a)In vivo 3D USI and PAI of a subcutaneous tumor in a mouse injected with gold nanorods. The subcutaneous tumor appears as a bump in the 3D ultrasound image. The PA image shows the heterogenous localization of nanorods in tumor, which preferentially accumulate there owing to the enhanced permeation and retention effect [69]. (b)In vivo PAI and OCT of the skin on the back of a nude mouse. The image represents a data fusion of OCT (structure of the skin) and PA (microvasculature) images. Adapted with permission from [64]. (c) Noninvasive in vivo fluorescence (FL) image acquired 24 hours after ICG injection in a mouse with melanoma cells implanted in brain. Noninvasive in vivo PA images were acquired with skin and skull intact, showing the vasculature in the brain. Adapted with permission from [70]. (d) Gray-scale ultrasound image (left), PA image (center), and elasticity (right) images of a tissue-mimicking phantom with a single inclusion. The inclusion had higher optical contrast and was harder compared to the background. (e) MMUS (top) and PA (bottom) images of a tissue-mimicking phantom with samples containing (left to right):a mixture of Fe3O4 nanoparticles and Au nanospheres; Au nanospheres only; PVA only (no nanoparticles); and Fe3O4 nanoparticles only. The MMUS colormap represents the displacement of the inclusions. The pure PVA sample (no nanoparticles) did not displace under magnetic excitation and showed no PA contrast. The Au nanospheres also do not displace, but have high optical absorption and hence produce greater PA signals compared with Fe3O4 nanoparticles. The two samples containing Fe3O4 nanoparticles had a displacement of about 100 µm [71].