Abstract
Background
So far, the neural network associated with posttraumatic stress disorder (PTSD) has been suggested to mainly involve the amygdala, hippocampus and medial prefrontal cortex. However, increasing evidence indicates that cortical regions extending beyond this network might also be implicated in the pathophysiology of PTSD. We aimed to investigate PTSD-related structural alterations in some of these regions.
Methods
We enrolled highly traumatized refugees with and without (traumatized controls) PTSD and non-traumatized controls in the study. To increase the validity of our results, we combined an automatic cortical parcellation technique and voxel-based morphometry.
Results
In all, 39 refugees (20 with and 19 without PTSD) and 13 controls participated in the study. Participants were middle-aged men who were free of psychoactive substances and consumed little to no alcohol. Patients with PTSD (and to a lesser extent traumatized controls) showed reduced volumes in the right inferior parietal cortex, the left rostral middle frontal cortex, the bilateral lateral orbitofrontal cortex and the bilateral isthmus of the cingulate. An influence of cumulative traumatic stress on the isthmus of the cingulate and the lateral orbitofrontal cortex indicated that, at least in these regions, structural alterations might be associated with repeated stress experiences. Voxel-based morphometry analyses produced largely consistent results, but because of a poorer signal-to-noise ratio, conventional statistics did not reach significance.
Limitations
Although we controlled for several important confounding variables (e.g., sex, alcohol abuse) with our particular sample, this might limit the generalizibility of our data. Moreover, high comorbidity of PTSD and major depression hinders a definite separation of these conditions in our findings. Finally, the results concerning the lateral orbitofrontal cortex should be interpreted with caution, as magnetic resonance imaging acquisition in this region is affected by a general signal loss.
Conclusion
Our results indicate that lateral prefrontal, parietal and posterior midline structures are implicated in the pathophysiology of PTSD. As these regions are particularly involved in episodic memory, emotional processing and executive control, this might have important implications for the understanding of PTSD symptoms.
Introduction
Posttraumatic stress disorder (PTSD) is a psychiatric condition that may emerge in reaction to a severe threat to life or bodily integrity. On a neuronal level, its symptom development has so far mainly been attributed to disturbed functioning of a network located in medial prefrontal and medial temporal lobe structures.1–3 Indeed, reports of PTSD-associated structural alterations within these regions have been numerous. Reduced volumes were reported for the hippocampus,4 amygdala4 and anterior cingulate cortex (ACC).5,6–8 Furthermore, a thinner prefrontal cortex has been shown in war veterans with chronic PTSD.9,10 Notwithstanding, it has repeatedly been highlighted that the network introduced above cannot satisfactorily account for the complex symptom pattern associated with the disease.11
Research in healthy individuals has revealed that the neuronal network mediating episodic memory and/or emotional processing (functions that are thought to be disturbed in PTSD) is widespread. Based on functional neuroimaging and brain lesion studies, the role of parietal,12,13 lateral prefrontal14–18 and posterior midline structures19 was particularly emphasized in this context. On a functional level, there is some evidence that these regions might be disturbed in PTSD. During trauma-related, script-driven imagery, increased neuronal activity was reported in retrosplenial and/or posterior cingulate,20 lateral prefrontal21 and parietal cortices.20,21 Furthermore, patients with PTSD showed an increased resting cerebral blood flow in posterior cingulate and parietal sections.22
On a structural level, PTSD-associated alterations in these cortical regions have received little attention so far. This might be partly owing to methodological problems with the evaluation of broader cortical regions. Manual segmentations are very time-consuming and not practicable for major sections. The alternative, classical automatic procedures would, on the other hand, not be accurate and sensitive enough to reveal the subtle structural alterations more typical for psychiatric conditions.23,24 Moreover, brain structural research on PTSD is impeded by the long-term pharmacological treatment and/or alcohol or substance abuse that is frequently associated with chronic PTSD.25 In particular, enduring and excessive alcohol consumption has repeatedly been shown to have a strong effect on brain structures and may thus distort findings.6,26
We aimed to investigate PTSD-related, structural alterations in cortical regions extending beyond the conventional psycho-biological model of this disease. In doing so, we chose specific regions of interest (ROIs) in prefrontal, parietal and posterior midline regions that have previously been associated with episodic memory12,14,19 and/or emotional processing,16–18 and we predicted that patients with PTSD should show reduced volumes in these structures. Furthermore, we speculated a “building-block effect” of traumatization, with greater cumulative exposure to traumatic stress leading to smaller brain volumes. As the currently most popular method of structural brain research, voxel-based morphometry (VBM), has recently come into question,23,24,27 we used 2 independent methods (a cortical parcellation technique and VBM) to improve the validity of our results. By choosing a study population that took no regular psychiatric medication and barely consumed alcohol, we controlled for confounding variables that often have hampered PTSD-related brain research.
Methods
Participants
We recruited participants from local shelters for asylum-seekers and Kurdish recreational facilities. Participants were included if they were healthy refugees between the ages of 18 and 55 years. Exclusion criteria were lifetime or current abuse of substances (particularly alcohol), neurologic diseases, any contraindication for magnetic resonance imaging (MRI) and psychiatric conditions other than PTSD or major depression.
The objective of the study was to investigate the effects of traumatization and PTSD on brain morphology. Accordingly, we explicitly screened participants for PTSD having developed as the primary disease in reaction to traumatic stress. In all participants, major depression had developed as a secondary, comorbid disease, and some fulfilled criteria for major depression according to DSM-IV.28 As sex influences on the results of morphometric analyses are well-documented,29,30 we selected a male sample to minimize the level of variability not owing to traumatization and/or PTSD. Our final sample comprised participants who currently had PTSD, participants who did not have PTSD but who had repeatedly experienced traumatic stress (traumatized controls) and nontraumatized controls who had not experienced severe traumatic stressors.
We conducted the investigation in 2 stages. At the first meeting, the purpose and the course of the investigation were explained in detail, informed consent was acquired, and diagnostic procedures took place. Magnetic resonance imaging measurements were obtained on a separate day (the time interval never exceeding 2 weeks) at the university hospital of Magdeburg, Germany. Participants received compensation of 70 euros. All procedures were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Konstanz, Germany.
Diagnostic interviews
Interviews were structured and administered in the maternal language of the participants with the aid of trained interpreters. Initially, sociodemographic information was obtained, and participants were questioned about their health status and smoking habits. Subsequent diagnostic procedures proceeded as follows.
vivo Checklist of war, detention and torture events
We evaluated exposure to traumatic stressors with a shortened version of the vivo Checklist of war, detention and torture events.31 The shortened scale is based on the unweighted sum of 28 imprisonment- and nonimprisonment-related traumatic event types (e.g., being beaten or receiving electrical shocks as imprisonment-related items, witnessing the murder of a relative or experiencing bombings as nonimprisonment-related items).
Clinician Administered PTSD Scale
We assessed current and lifetime PTSD symptoms with the Clinician Administered PTSD Scale (CAPS32). This 30-item, structured interview corresponds to PTSD criteria according to DSM-IV28 and allows a quantification of the 3 clusters of PTSD symptoms (intrusions, avoidance and hyperarousal).
Mini-International Neuropsychiatric Interview
The diagnosis of major depression, suicidal ideations and alcohol or substance dependency or abuse according to DSM-IV28 was based on the corresponding sections of the Mini-International Neuropsychiatric Interview (MINI).33
MRI acquisition and data analyses
High-resolution, whole-brain, 3-dimensional (3-D) structural MRI scans were acquired on a 3 T Siemens MAGNETOM Trio scanner with an 8-channel phased-array head coil using a T1-weighted 3D–magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence in sagittal orientation (echo time [TE] 4.77 ms, repetition time [TR] 2500 ms, TI 1100 ms, flip angle 7°, bandwidth 140 Hz/pixel, matrix 256 × 256 × 192, field of view [FOV] 256 mm, isometric voxel size 1.0 mm3).
FreeSurfer cortical parcellation and volume measurements
We performed cortical reconstruction and volumetric segmentation with the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/). The precise technical details of these procedures are described elsewhere.34,35 In short, each scan is registered into Talairach space, intensity-corrected and skull-stripped. Images are then segmented to identify the boundary between grey and white matter and to create a surface representation of the cortical white matter. Finally, the cerebral cortex is parcellated into units based on its gyral and sulcal structure.36 According to probabilistic information estimated from a reference atlas, a neuroanatomical label is assigned to each vertex of the surface model, and the corresponding information (i.e., volume) is calculated for each section. All procedures with FreeSurfer are conducted in native space.
The quality of the skull-stripping and the accuracy of the grey/white matter boundary as well as the pial surface were reviewed by an anatomically skilled operator, who was blind to any group membership. If necessary, results of the surface reconstruction were edited manually. The following regions that have previously been associated with episodic memory12,14,19 and/or emotional processing16–18 were chosen for further analysis:
prefrontal cortex (superior frontal cortex, rostral middle frontal cortex, inferior frontal cortex, orbitofrontal cortex and ACC),
posterior midline structures (posterior cingulate cortex, isthmus of the cingulate, precuneus), and
lateral parietal cortex (superior parietal cortex, inferior parietal cortex and supramarginal cortex).
Voxel-based morphometry
As specific preprocessing steps may enhance the accuracy of VBM,37 MRI scans were skull-stripped with BET238 and bias-corrected39 before analyses. Subsequent VBM analyses were performed using SPM5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London) running in MAT-LAB R2006a (Mathworks). Magnetic resonance images were spatially normalized and then segmented based on their intensity distribution and spatial information derived from prior probability maps.40 To keep our analysis comparable to previous VBM in patients with PTSD,5–7 we smoothed the images with a 12-mm full-width at half-maximum isotropic Gaussian kernel. As the VBM analysis further aimed to replicate the previous cortical parcellation analysis, we focused on the ROIs that have been included in the cortical parcellation analysis. Bilateral ROIs were created based on an average participant (the so-called Bert) provided by FreeSurfer and were then normalized in Montreal Neurological Institute (MNI) space and smoothed with the identical parameters as the participants’ MRI scans. Subsequent statistical VBM analyses were masked for the ROIs under investigation.
Statistical analysis
Sample characteristics
We compared sample characteristics and clinical parameters using analyses of variance (ANOVAs). All data were tested for normality with the Shapiro–Wilk test.41 If the normality assumption was not fulfilled, we calculated nonparametric alternatives (Kruskal–Wallis rank sum tests). For post-hoc comparisons, we used pair-wise t tests and, as a nonparametric alternative, pair-wise Wilcoxon rank sum tests. Post-hoc tests were corrected for multiple comparisons according to Hommel.42 We analyzed count data using Fisher exact tests.
Cortical parcellation
As age and intracranial volume (ICV) are potential confounds for volumetric measures of brain structures,28 we considered these 2 parameters as covariates in all structural analyses. Volumetric group differences were analyzed with linear mixed-effects models, in which hemisphere was included as a within-group factor. Specific group differences were clarified by inspection of the corresponding parameter estimates in the linear mixed-effects models. If a significant group × hemisphere interaction (indicating a lateralized group effect) was revealed, each hemisphere was considered separately in a linear model. To control for an effect of lifetime PTSD on volumetric variables, analyses were repeated under exclusion of participants with a diagnosis of lifetime PTSD.
Voxel-based morphometry
We initially explored group differences in SPM5, applying a full factorial model with age and intracranial volume as covariates. Directional t contrasts were defined between groups. The corresponding SPM(t) values were transformed to the normal distribution (SPM(z)) and thresholded at p < 0.005 (uncorrected) with a minimum cluster size of 25 voxels. We extracted mean intensity values in the encountered clusters using MarsBaR.43 Intensity values for each cluster were then directly compared in linear models, again including age and intracranial volume as covariates.
Effects of cumulative exposure to traumatic stress
We investigated a putative dosage effect of multiple traumatic event types on the probability of PTSD diagnosis for traumatized participants using a logistic regression model. The effect of the number of different traumatic events on PTSD symptom severity was explored using a bivariate regression model. To reveal a possible relation between the severity of trauma exposure and parcellation results/mean intensity values, these variables were included in a linear regression model and corrected for age and intracranial volume as covariates. Models were then compared with likelihood ratio tests. We considered the number of traumatic stress types experienced to be influential if the model including trauma exposure was favoured.
We performed all analyses (except the exploration of VBM group differences in SPM5) using the statistical program R (version 2.7.144) with the additional package nlme (version 3.1–9045).
Results
Participants
Fifty-two refugees were included in the study: 20 currently had PTSD, 19 did not have PTSD but had repeatedly experienced traumatic stress (traumatized controls) and 13 non-traumatized controls had not experienced severe traumatic stressors. In 3 of the traumatized controls, an earlier episode of PTSD had remitted.
The main population characteristics of the sample are summarized in Table 1. Participants’ mean age was 36 years in the PTSD group (standard deviation [SD] 7.7, range 23–55 yr), 34 years in traumatized controls (SD 9.9, range 21–53 yr) and 29 years in nontraumatized controls (SD 7.2, range 18–48 yr). The group difference regarding age reached significance: Kruskal–Wallis χ22 = 7.35, p = 0.025. Post-hoc tests revealed that nontraumatized controls were younger than participants with PTSD (Wilcoxon rank sum test, p = 0.010). However, nontraumatized controls did not differ significantly from traumatized controls, and traumatized controls did not differ from patients with PTSD. In an attempt to control for this confound, age was considered as covariate in every subsequent analysis. Groups tended to differ regarding the years of formal education: Kruskal–Wallis χ22 = 5.06, p = 0.08. Post-hoc tests revealed that traumatized controls tended to have had more years of formal education than patients with PTSD (Wilcoxon rank sum test, p = 0.06). Participants were mainly of Kurdish (n = 48) race. The 4 remaining participants were Albanian, Serbian, Romanian and Turkish, respectively. Forty-nine participants were right-handed, and 3 participants (1 in each control group and 1 patient with PTSD) were left-handed. One participant in the PTSD group had taken antidepressant medication on an irregular basis (maximally once a week). Twenty-nine participants were smokers: 9 nontraumatized controls (mean 18.00, SD 6.40 cigarettes/d), 10 traumatized controls (mean 22.18, SD 13.66 cigarettes/d) and 10 patients with PTSD (mean 23.10, SD 16.04 cigarettes/d). Group differences in the number of smokers or cigarettes smoked per day were nonsignificant. Other than that, none of the participants consumed any psychoactive drugs or medication.
Table 1.
Population characteristics of refugees who underwent magnetic resonance imaging to assess the effects of traumatization and PTSD on brain morphology
| Group; mean (SD)* |
|||||
|---|---|---|---|---|---|
| Characteristic | Nontraumatized controls, n = 13 | Traumatized controls, n = 19 | PTSD, n = 20 | Kruskal–Wallis χ22† | p value† |
| Age, yr | 29.0 (7.2) | 34.1 (9.9) | 36.2 (7.7) | 7.4 | 0.025 |
| Years of formal education | 8.5 (6.0) | 10.7 (4.4) | 7.6 (4.0) | 5.1 | 0.08 |
| Cigarettes smoked, no./d | 12.5 (10.1) | 12.8 (15.2) | 11.6 (16.2) | 0.75 | |
| Age at first traumatic experience | — | 15.5 (6.8) | 16.4 (6.8) | 0.86 | |
| No. smokers | 9 | 11 | 9 | 0.38 | |
| No. participants fulfilling criteria for major depression | 1 | 1 | 15 | < 0.001 | |
PTSD = posttraumatic stress disorder; SD = standard deviation.
Unless otherwise indicated.
All test results were 2-tailed.
Most of the traumatized participants were exposed to severe traumatic stress more than a decade ago: 44% reported their first traumatic event 10–20 years ago; 41% reported that traumatic experiences had started even more than 20 years ago. Participants were between 5 and 35 years old when they experienced their first traumatic event (mean age 15.8, SD 6.6 yr). Patients with PTSD and traumatized controls did not differ regarding their age at first traumatic experience. As expected, patients with PTSD reported experiencing a greater number of different types of traumatic events (see Table 2 for means and SDs of clinical instruments in traumatized participants). Seventeen participants (1 in each control group and 15 in the PTSD group) fulfilled criteria for major depression according to DSM-IV.28 Eleven participants showed either low (n = 10) or high (n = 1) suicidality, with higher suicidality in participants with PTSD: Pearson χ24 = 11.26, p = 0.024.
Table 2.
Traumatization and symptoms of posttraumatic stress disorder
| Group; mean (SD)
|
||||
|---|---|---|---|---|
| Measure | Traumatized controls, n = 19 | PTSD, n = 20 | Kruskal–Wallis χ21* | p value* |
| Checklist | 7.68 (4.66) | 14.80 (5.63) | 13.26 | < 0.001 |
| CAPS score | ||||
| Sum of event list | 4.68 (2.24) | 6.60 (2.19) | 5.53 | < 0.001 |
| Intrusion subscale | 7.05 (5.19) | 22.70 (6.14) | 25.66 | < 0.001 |
| Avoidance subscale | 3.16 (4.95) | 26.10 (6.10) | 27.42 | < 0.001 |
| Hyperarousal subscale | 2.84 (4.22) | 20.10 (5.99) | 25.42 | < 0.001 |
| Sum | 13.05 (11.98) | 68.90 (15.46) | 27.04 | < 0.001 |
Of the 52 participants, 3 (1 in each group) were excluded from further analysis because their MRI data were of extremely bad quality owing to movement artifacts.
Group differences in cortical volume and cerebral grey matter
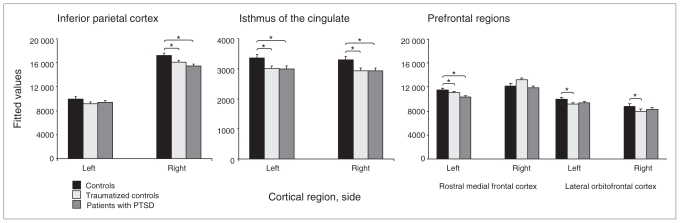
See Figure 1 for a graphic depiction of cortical parcellation results and Table 3 for a succinct summary of corresponding statistical models. No significant group differences were found regarding the cortex as a whole (F2,44 = 0.53, p = 0.59) or total grey matter (F2,44 = 0.42, p = 0.66). However, groups differed in the bilateral isthmus of the cingulate (F2,44 = 3.98, p= 0.026). Compared with the nontraumatized controls, the PTSD group (t44 = −2.59, p = 0.013) and the traumatized controls (t44 = −2.48, p = 0.017) showed lower volumes in this section. Traumatized controls and patients with PTSD did not differ significantly (t44 = −0.04, p = 0.97). Furthermore, there was a trend toward a bilateral group difference in the lateral orbitofrontal cortex (F2,44 = 2.38, p = 0.10). Traumatized controls showed less volume than nontraumatized controls (t44 = −2.17, p = 0.035). However, the difference between non-traumatized controls and patients with PTSD (with less volume in the PTSD group) did not reach statistical significance (t44 = −1.49, p = 0.14). Again, traumatized controls and the PTSD group did not differ (t44 = 0.84, p = 0.41).
Fig. 1.
Graphic depiction of group differences in cortical regions associated with episodic/autobiographical memory. Depicted are the fitted values (predicted group means with the covariates kept constant at the mean of the whole population) and standard errors (original uncorrected volumes were given in millimetres). Significant group differences were found in the bilateral isthmus of the cingulate, the left rostral middle frontal cortex and the right inferior parietal cortex. The bilateral lateral orbitofrontal cortex showed a trend toward group differences. Age and intracranial volume were considered as covariates in all analyses. Precise statistical parameters are presented within the main text. MFC = medial frontal cortex; OFC = orbitofrontal cortex; PTSD = posttraumatic stress disorder.
Table 3.
Summary of group differences in cortical volume and cerebral grey matter
| Parameter statistics* |
Covariate statistics* |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NTC v. PTSD
|
NTC v. TC
|
TC v. PTSD
|
Age
|
ICV
|
Hemisphere
|
|||||||||
| Imaging; brain region | F2,44 | p value | t44 | p value | t44 | p value | t44 | p value | F1,44 | p value | F1,44 | p value | F1,48 | p value |
| Parcellation | ||||||||||||||
| Bilateral isthmus of the cingulate | 3.98 | 0.025 | −2.59 | 0.013 | −2.48 | 0.017 | −0.04 | 0.97 | 14.66 | < 0.001 | 41.65 | < 0.001 | 1.12 | 0.30 |
| Bilateral lateral orbitofrontal cortex | 2.38 | 0.10 | −1.49 | 0.14 | −2.17 | 0.035 | 0.84 | 0.41 | 9.02 | 0.004 | 13.57 | < 0.001 | 123.95 | < 0.001 |
| Left rostral middle frontal cortex | 4.12 | 0.022 | −2.68 | 0.010 | −0.84 | 0.40 | −2.03 | 0.048 | 8.33 | 0.006 | 6.82 | 0.012 | — | |
| Right inferior parietal cortex | 4.57 | 0.016 | −3.02 | 0.004 | −1.90 | 0.06 | −1.20 | 0.24 | 4.92 | 0.031 | 28.22 | < 0.001 | — | |
| VBM grey matter volumes (extracted with MarsBaR) | — | |||||||||||||
| Left isthmus of the cingulate | 5.45 | 0.008 | −3.26 | 0.002 | −2.41 | 0.020 | −0.87 | 0.39 | 3.69 | 0.06 | 16.46 | < 0.001 | — | |
| Right inferior parietal cortex | 6.69 | 0.003 | −3.65 | < 0.001 | −2.03 | 0.049 | −1.75 | 0.09 | 12.93 | < 0.001 | 9.62 | 0.003 | — | |
| Left rostral anterior cingulate cortex | 4.75 | 0.013 | −3.03 | 0.004 | −2.30 | 0.026 | −0.75 | 0.46 | 7.34 | 0.010 | 10.59 | 0.002 | — | |
| Right rostral anterior cingulate cortex | 6.01 | 0.005 | −3.24 | 0.002 | −2.96 | 0.005 | −0.22 | 0.83 | 3.29 | 0.08 | 12.20 | 0.001 | — | |
ICV = intracranial volume; NTC = nontraumatized control group; PTSD = posttraumatic stress disorder; TC = traumatized control group; VBM = voxel-based morphometry.
All tests were 2-tailed, with p < 0.001 indicating a significant group difference.
We found significant group × hemisphere interactions in the rostral middle frontal cortex (F2,46 = 4.59, p = 0.015) and inferior parietal cortex (F2,46 = 4.39, p = 0.018). Therefore, volumes were compared separately for each hemisphere in these regions. In the rostral middle frontal cortex, we found a significant group difference in the left hemisphere (F2,44 = 4.12, p = 0.023). Participants with PTSD showed lower volumes than both control groups (nontraumatized controls v. PTSD, t44 = −2.68, p = 0.010; traumatized controls v. PTSD, t44 = −2.03, p = 0.048; nontraumatized v. traumatized controls, t44 = −0.84, p = 0.40). In the inferior parietal cortex, there was a significant right-hemispheric difference (F2,44 = 4.57, p = 0.015). In this case, patients with PTSD as well as traumatized controls showed lower volumes than nontraumatized controls (non-traumatized controls v. PTSD, t44 = −3.02, p = 0.004; traumatized controls v. PTSD, t44 = −1.20, p = 0.24; nontraumatized v. traumatized controls, t44 = −1.90, p = 0.06). Excluding traumatized controls who fulfilled the criteria of a lifetime PTSD or left-handed persons did not affect the results.
Voxel-based morphometry grey matter volume
See Figure 2 for a graphic depiction of VBM results and Table 3 for a summary of corresponding statistical models. At the uncorrected significance threshold of p < 0.005 (minimum cluster size [k] of 25 voxels), clusters with lower grey matter volumes in patients with PTSD than nontraumatized controls were found in the vicinity of the left isthmus of the cingulate (peak coordinates x, y, z mm = −10, −48, 28; k = 111, t = 3.35), the right inferior parietal cortex (peak coordinates x, y, z mm = 30, −80, 32 and 34, −80, 20; k = 175, t = 3.43 and 3.08) and the bilateral rostral ACC (peak coordinates x, y, z mm = −14, 44, 14; k = 57, t = 3.38 in the left hemisphere and 16, 40, 16; k = 36, t = 3.08 in the right hemisphere). No significant differences were observed comparing healthy and traumatized controls or traumatized controls and patients with PTSD.
Fig. 2.
Brain regions showing less grey matter volume in patients with posttraumatic stress disorder (PTSD) than in nontraumatized controls (at a threshold of p < 0.005, uncorrected). Results of the voxel-based morphometry (VBM) analysis did not reach significance within a classic voxel-wise comparison. Bar graphs depict the fitted values (predicted group means with the covariates kept constant at the mean of the whole population) and standard errors of extracted mean volume levels in the respective clusters. After extraction of mean volume levels, significant group differences were found in the inferior parietal cortex (patients with PTSD and traumatized controls showed significantly lower grey matter volume than nontraumatized controls and, as a trend, patients with PTSD showed lower grey matter volumes than traumatized controls), isthmus of the cingulate (patients with PTSD and traumatized controls showed significantly less grey matter volume than nontraumatized controls) and bilateral anterior cingulate cortex (patients with PTSD and traumatized controls showed significantly lower grey matter volume than nontraumatized controls). Precise statistical parameters are presented within the main text.
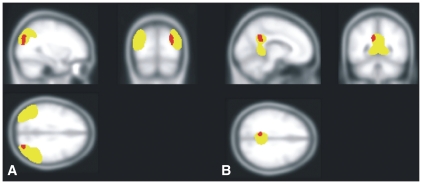
In a direct comparison of the mean volumes extracted with MarsBaR, group differences reached significance in all SPM clusters: in the vicinity of the left isthmus of the cingulate, patients with PTSD and traumatized controls showed less grey matter volume than nontraumatized controls (F2,44 = 5.45, p = 0.008; nontraumatized controls v. PTSD, t44 = −3.26, p = 0.002; traumatized controls v. PTSD, t44 = −0.87, p = 0.39; nontraumatized v. traumatized controls, t44 = −2.41, p = 0.020). In the right inferior parietal cortex, traumatized participants showed less grey matter volume than nontraumatized controls (F2,44 = 6.69, p = 0.003; nontraumatized controls v. PTSD, t44 = −3.65, p < 0.001; nontraumatized v. traumatized controls, t44 = −2.03, p = 0.049). Furthermore, there was a trend with traumatized controls showing less grey matter volume than patients with PTSD (t44 = −1.75, p = 0.09). In the bilateral rostral ACC, patients with PTSD and traumatized controls showed lower grey matter volumes than nontraumatized controls (left hemisphere: F2,44 = 4.75, p = 0.014; nontraumatized controls v. PTSD, t44 = −3.03, p = 0.004; traumatized controls v. PTSD, t44 = −0.75, p = 0.46; nontraumatized v. traumatized controls, t44 = −2.30, p = 0.026; right hemisphere: F2,44 = 6.01, p = 0.005; nontraumatized controls v. PTSD, t44 = −3.24, p = 0.002; traumatized controls v. PTSD, t44 = −0.22, p = 0.83; nontraumatized v. traumatized controls, t44 = −2.96, p = 0.005). See Figure 3 for a graphic depiction of the VBM results and the underlying, smoothed ROIs generated based on the FreeSurfer parcellation.
Fig. 3.
Graphic depiction of the overlap between the 2 analysis methods in (A) the inferior parietal cortex and (B) the isthmus of the cingulate. Brain regions showing lower grey matter volumes in patients with posttraumatic stress disorder than in nontraumatized controls (at a threshold of p < 0.005, uncorrected) are depicted in red. The underlying, smoothed regions of interest generated based on the FreeSurfer parcellation are depicted in yellow.
Building-block effect
We found a strong positive relation between the number of traumatic event types experienced by a participant and the incidence of PTSD ([log P(PTSD) ÷ P(1-PTSD)] = −3.10 + 0.28 × vivo Checklist; R2adj = 15.81, p < 0.001). Furthermore, a linear regression analysis showed a significant relation between cumulative exposure to traumatic stress and current symptom severity of PTSD (CAPS sum 4.35 + 3.22 × vivo Checklist; R2adj = 0.37, ANOVA F1,35 = 21.91, p < 0.001).
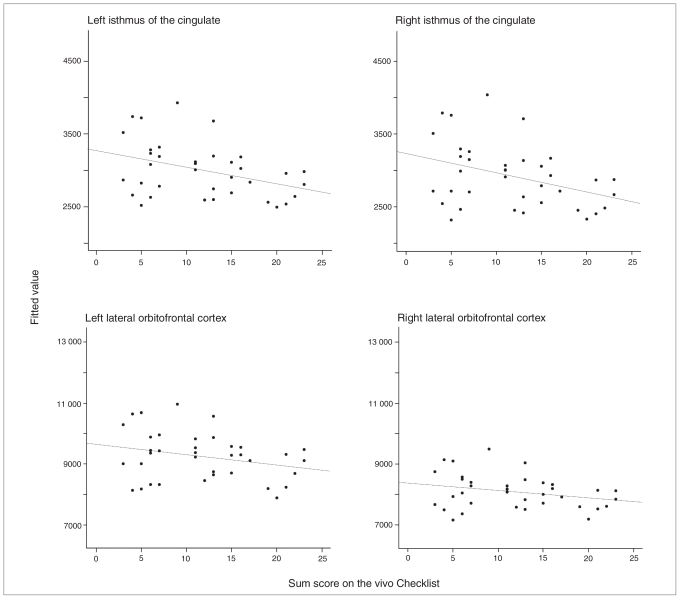
Likelihood ratio tests supported a significant influence of the sum score of traumatization in the isthmus of the cingulate (χ22 = 5.92, p = 0.05). Furthermore, an influence was revealed in the lateral orbitofrontal cortex (χ22 = 8.09, p = 0.018). In both cases, this effect was mediated by intracranial volume (isthmus of the cingulate: ICV × vivo Checklist: t32 = −2.35, p = 0.026; lateral orbitofrontal cortex: ICV × vivo Checklist: t32 = −2.73, p = 0.010). See Figure 4 for a graphic depiction of the relation between the extent of traumatization and brain volumes and Table 4 for the model equations and respective parameter statistics. No influence of traumatization could be shown for the left rostral middle frontal cortex and the right inferior parietal cortex. The influence of the sum score of traumatization on parcellation variables could not be replicated for mean volume levels in the respective clusters of the VBM.
Fig. 4.
Correlation between the extent of traumatization and brain volumes. There was a significant relation between the extent of traumatization, the bilateral isthmus of the cingulate and the bilateral lateral orbitofrontal cortex. Scatter plots depict fitted values (predicted group means with the covariates kept constant at the mean of the whole population) of brain volumes. Precise statistical parameters are presented within the main text.
Table 4.
Influence of traumatization on cortical volumes
| Parameter statistics
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age
|
Hemisphere
|
ICV
|
Group
|
vivo Checklist
|
ICV × vivo Checklist
|
|||||||
| Brain region | t31 | p value | t36 | p value | t31 | p value | t31 | p value | t31 | p value | t31 | p value |
| Bilateral isthmus of the cingulate* | −2.18 | 0.037 | 1.12 | 0.27 | 4.92 | < 0.001 | 0.64 | 0.53 | 2.38 | 0.024 | −2.35 | 0.026 |
| Bilateral lateral orbitofrontal cortex† | −1.81 | 0.08 | 9.91 | < 0.001 | 4.66 | < 0.001 | −0.78 | 0.44 | 2.67 | 0.012 | −2.68 | 0.012 |
ICV = intracranial volume; vivo Checklist = shortened version of the vivo Checklist of war, detention and torture events.31
Model equation: Isthmus of the cingulate = −2988 − 13.69 × age + 83.95 × hemisphere + 0.003 × ICV + 87.29 × group + 272.12 × vivo Checklist − 0.0001 × ICV × vivo Checklist.
Model equation: Lateral orbitofrontal cortex = −4677 − 26.71 × age + 1162.27 × hemisphere + 0.007 × ICV −248.09 × group + 716.75 × vivo Checklist − 0.0001 × ICV × vivo Checklist.
Discussion
The scope of the present study was to investigate the influence of traumatization and PTSD on cortical grey matter volumes. To increase the validity of our findings, we implemented 2 independent methods: an automated cortical parcellation analysis and VBM. According to the cortical parcellation, patients with PTSD (and to a lesser extent traumatized controls) showed reduced brain volumes within several lateral prefrontal regions, the right inferior parietal cortex and the bilateral isthmus of the cingulate. Subsequent regression analysis revealed that this volume loss correlated with the extent of traumatization at least in lateral orbitofrontal cortices and the isthmus of the cingulate. These results were partially confirmed by the VBM analysis, showing a PTSD-related decrease of grey matter volumes in the right parietal cortex, left posterior midline regions and, beyond the parcellation findings, in the bilateral rostral ACC. However, VBM results did not survive conventional correction for multiple comparisons and should therefore generally be interpreted with caution.
So far, etiological concepts of PTSD considered its symptom pattern to be mainly associated with alterations in medial pre-frontal and medial temporal lobe regions.1–3 Support for this notion came from numerous studies reporting reduced volumes in the hippocampus,4 amygdala,4 prefrontal cortex9,10 and ACC.5–8 However, it has repeatedly been stated that these structures cannot account for all symptoms and deficits observed.11 By demonstrating respective volume reductions within lateral prefrontal, parietal and posterior midline structures the present results provide evidence that these areas might be indeed implicated in PTSD and/or traumatization.
Reports of PTSD- and/or stress-related structural and functional alterations in prefrontal regions are numerous. Besides the previously mentioned volume reductions in the ACC5–8 and lateral prefrontal cortex,9,10 patients with PTSD showed altered brain functions in reaction to trauma-related memories in both regions.20,21,46 Moreover, a disturbed ability of traumatized individuals to down-regulate negative emotional responses was directly associated with reduced brain activity in the lateral prefrontal cortex.47 Respective alterations might emerge very early in reaction to traumatic stress, as survivors of a severe earthquake showed an increased resting-state activity in the left lateral prefrontal cortex shortly after having experienced of this traumatic event.48
It has recently been highlighted that periods of repeated (psychosocial) stress might alter the activity in the human pre-frontal cortex.49 In light of corresponding findings of stress-induced dendritic atrophy in rodents,50 these processes might manifest themselves in detectable structural alterations when extreme and/or repeated traumatic stress is experienced. Support for this notion might come from the association between the extent of traumatization and the volumes of the lateral orbitofrontal cortex that has been revealed in our data. Substantial volume loss in the lateral orbitofrontal cortex was already reported in war veterans with chronic PTSD.10 We were able to replicate this finding and add (in line with insights from research in rodents50 and reports of the consequences of severe psychosocial49 and traumatic48 stress on prefrontal brain functions in humans) that this volume loss might be interpreted as a consequence of repeated traumatic stress.
As a potential contribution of parietal and/or posterior midline structures has received relatively little attention in trauma and/or PTSD-related brain research so far, data concerning this topic are still scarce. However, there is some evidence in the literature that supports our suggestion that these structures might play some role in the development of PTSD symptoms as well. During trauma-related, script-driven imagery, an increased neuronal activity was reported in retrosplenial and/or posterior cingulate20 and parietal cortices20,21 of patients with PTSD. Furthermore, patients with PTSD showed an increased resting cerebral blood flow in posterior cingulate and parietal sections.22
Taken together, our results and those in the literature point out that lateral prefrontal, parietal and posterior midline regions might be involved in the pathophysiological model of PTSD. A congruency of the cortical parcellation and VBM analysis, at least in some of the regions, provides further support for the validity of this finding. A previous combination of these 2 methods51 in traumatized participants revealed highly consistent results between the FreeSurfer parcellation and VBM. These authors implemented the cortical parcellation method to validate their whole-brain VBM analysis.51 However, we chose to apply the methods in an opposing order. Apart from its popularity in clinical research, there has been emerging concern about some limitations of VBM. Criticism mainly concentrated on a potential distortion of results owing to spatial normalization,27 a bias toward group differences that are spatially well confined24 and statistical procedures that may generally be too strict to reveal subtle morphological alterations.23 FreeSurfer procedures are, on the other hand, performed in native space, thus avoiding spatial normalization steps that might distort findings. Intersubject and/or template registrations are performed by projecting them onto spherical representations. This approach has been shown to result in a good matching of homologous cortical regions and should thus be more sensitive than classic VBM.
In line with these preceding considerations, our VBM results generally did not survive conventional correction for multiple comparisons, even though they tended to indicate atrophies in similar regions as the cortical parcellation. Moreover, the results differed between methods for some other brain regions, for example, in the lateral prefrontal cortex where VBM failed to replicate a volume loss that has been revealed with the parcellation method. However, as mentioned previously, it has been suggested that VBM findings might be distorted by normalization steps.24,27 As these nuisance effects might be especially pronounced at the edges of the brain, this might help to explain some of these inconsistencies. Moreover, VBM revealed PTSD-related structural alterations in the rostral ACC that were not observed with the parcellation procedure. This parallels previous reports in the literature7 and emphasizes the notion that VBM is not sufficiently able to differentiate between factual volume loss and alterations in shape and/or location of brain structures.24 To summarize, our data indicate that FreeSurfer and VBM are both suitable for the investigation of cerebral atrophies. However, our results still support some of the concerns mentioned above23,24,27 and imply that VBM should be combined with other methods to increase its informative value.52
Limitations
Some major limitations should be considered when interpreting the present results. Our study population consisted of mainly Kurdish, male refugees exposed to similar severe traumatic experiences in their home countries. As this specific population took no regular psychiatric medication and barely consumed alcohol, we controlled for confounding variables that frequently have hampered PTSD-related brain research. Nevertheless, this sample leads to a limited generalizibility of our findings, as conclusions about potential sex differences or the impact of different kinds of traumatization (e.g., childhood abuse) cannot be drawn. As most of our participants had comorbid major depression, we furthermore cannot definitely distinguish how PTSD and depression symptoms contributed to our results. However, in light of the high prevalence of comorbid major depression in patients with PTSD, it has already been suggested that major depression and PTSD symptoms might emerge simultaneously as 2 facets of a general posttraumatic psychopathology.5,53 Accordingly, the strict division between these 2 conditions might be artificial and not representative of the factual clinical reality in chronic PTSD.
Another line of concern affects general methodological issues. We had to calculate 12 independent statistical models to investigate the effects of PTSD and/or traumatization on our hypothesized ROIs. However, as we did not directly correct for multiple comparisons within this procedure, we cannot definitely rule out the possibility of false-positive results. However, given the mentioned 12 tests covering our parcellation ROIs, we would expect at most 1 random deviation on a 0.05 significance level. Our finding of 4 regions differing between groups thus largely exceeds the expectations of mere chance. Moreover, our a priori hypotheses were 1-sided, which would allow us to divide the respective p values by 2, thus further strengthening the group differences revealed in our data. Finally, the validity of our findings was further increased by the implementation of 2 independent methods providing an overlapping pattern of results.
Another methodological concern that might limit the interpretation of our results is linked to general constraints of MRI acquisition. We revealed a significant volume loss in the lateral orbitofrontal cortex that was associated with the extent of traumatization. However, MRI acquisition is generally plagued by signal loss in this region. It is hard to quantify or control for the influence of this nuisance factor on our results. Notwithstanding, acquisition parameters have been identical for all participants and individuals have been scanned in an interleaved manner. Accordingly, the measurement error in the lateral orbitofrontal cortex should be constant for the whole population and should not have a systematically bigger effect in one group than the other. Further support for this line of argument comes from the literature. Similar volumetric differences in the lateral orbitofrontal cortex have already been shown in a relatively large sample of former Vietnam veterans,10 and it seems highly improbable that MRI nuisance artifacts affected 2 completely independent populations in the same direction.
Conclusion
Apart from the concerns mentioned, our findings on PTSD-and/or trauma-related structural alterations in lateral prefrontal, parietal and posterior midline regions might have important implications for the understanding of PTSD symptoms and some associated memory disturbances. These regions are part of a network that is particularly involved in episodic memory, emotional processing and executive control. Prefrontal regions play a particular role in the deliberate manipulation of emotions16,17,47 and memories14,15,54 and might thus be particularly important for the regulation of highly emotional memories in the aftermath of traumatic experiences.55 The parietal cortex, on the other hand, has been suggested to play an important role in the volitional and unvolitional allocation of attentional resources12,13 during the retrieval of episodic memories. Accordingly, the successful manipulation of emotional memories seems not only to rely on the interplay between medial temporal and prefrontal cortices but also on an intact functioning of parietal areas. Integrity of the posterior midline structures might finally be particularly important for an unobstructed communication between these structures, as this region is known to serve as a major route of information flow between them.56 Disturbances in this hypothesized network, as they are indicated by our data, might help to explain some of the memory disturbances associated with PTSD, such as the fragmentation of traumatic memories,1,2 the generally less detailed retrieval of autobiographical memories57 or the high occurrence of recurrent, intrusive recollection of traumatic memories. It must be emphasized, however, that this interpretation remains largely speculative. Even though we presented clear evidence that lateral prefrontal, parietal and posterior midline structures might be implicated in the pathophysiology of PTSD, the factual significance of these regions in PTSD symptom development still remains to be clarified.
Footnotes
Competing interests: This research was supported by the German Research Foundation (DFG); the DFG had no further role in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the paper for publication. None of the authors reported any biomedical financial interest or potential conflicts of interest.
Contributors: All authors contributed to study design and approved the article’s publication. Dr. Eckart recruited and assessed the participants. Drs. Eckart, Kaufmann and Tempelmann gathered MRI data. Drs. Eckart, Stoppel, Kaufmann and Tempelmann analyzed the data. Drs. Eckart and Stoppel wrote the initial draft of the manuscript, which was critically reviewed by Drs. Elbert and Kolassa. All authors discussed the results and implications and commented on the manuscript at all stages.
References
- 1.Brewin CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav Res Ther. 2001;39:373–93. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 2.Kolassa I-T, Elbert T. Structural and functional neuroplasticity in relation to traumatic stress. Curr Dir Psychol Sci. 2007;16:321–5. [Google Scholar]
- 3.Elbert T, Schauer M. Burnt into memory. Nature. 2002;419:883. doi: 10.1038/419883a. [DOI] [PubMed] [Google Scholar]
- 4.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Kasai K, Yamasue H, Gilbertson MW, et al. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–6. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–43. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbo V, Clement MH, Armony JL, et al. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry. 2005;58:119–24. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–7. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Geuze E, Westenberg HG, Heinecke A, et al. Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage. 2008;41:675–81. doi: 10.1016/j.neuroimage.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Woodward SH, Schaer M, Kaloupek DG, et al. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1373–82. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- 11.Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AD, Shannon BJ, Kahn I, et al. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Cabeza R, Ciaramelli E, Olson IR, et al. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–9. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MC, Ochsner KN, Kuhl B, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 16.Blair KS, Smith BW, Mitchell DG, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochsner KN. Current directions in social cognitive neuroscience. Curr Opin Neurobiol. 2004;14:254–8. doi: 10.1016/j.conb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Cardinal RN, Parkinson JA, Hall J, et al. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 19.Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44:1188–200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piefke M, Pestinger M, Arin T, et al. The neurofunctional mechanisms of traumatic and non-traumatic memory in patients with acute PTSD following accident trauma. Neurocase. 2007;13:342–57. doi: 10.1080/13554790701851494. [DOI] [PubMed] [Google Scholar]
- 21.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–11. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 22.Semple WE, Goyer PF, McCormick R, et al. Attention and regional cerebral blood flow in posttraumatic stress disorder patients with substance abuse histories. Psychiatry Res. 1996;67:17–28. doi: 10.1016/0925-4927(96)02735-7. [DOI] [PubMed] [Google Scholar]
- 23.Bergouignan L, Chupin M, Czechowska Y, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–90. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 26.Woodward SH, Kaloupek DG, Streeter CC, et al. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163:674–81. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- 27.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images”. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): The Association; 1994. [Google Scholar]
- 29.Pell GS, Briellmann RS, Chan CH, et al. Selection of the control group for VBM analysis: influence of covariates, matching and sample size. Neuroimage. 2008;41:1324–35. doi: 10.1016/j.neuroimage.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 30.Lochhead RA, Parsey RV, Oquendo MA, et al. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–62. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Schauer M, Neuner F, Elbert T. Narrative exposure therapy. 2nd ed. Göttingen (Germany): Hogrefe & Huber; 2011. [Google Scholar]
- 32.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 34.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 36.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Acosta-Cabronero J, Williams GB, Pereira JM, et al. The impact of skull-stripping and radio-frequency bias correction on grey-matter segmentation for voxel-based morphometry. Neuroimage. 2008;39:1654–65. doi: 10.1016/j.neuroimage.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh Annual Meeting of the Organization for Human Brain Mapping; 2005 June 12–16; Toronto, Ont. [Google Scholar]
- 39.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 40.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 42.Hommel G. A comparison of two modified Bonferroni procedures. Biometrika. 1989;76:624–5. [Google Scholar]
- 43.Brett M, Anton J-L, Valabregue R, et al. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; 2002 June 2–6; Sendai, Japan. [Google Scholar]
- 44.R Development Core Team. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2007. [Google Scholar]
- 45.Pinheiro J, Bates D, DebRoy S, et al. Nlme: Linear and nonlinear mixed effects models. R package version 3. Vienna (Austria): R Foundation for Statistical Computing; 2008. pp. 1–90. [Google Scholar]
- 46.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.New AS, Fan J, Murrough JW, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–64. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Lui S, Huang X, Chen L, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci U S A. 2009;106:15412–7. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 51.Tomoda A, Navalta CP, Polcari A, et al. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol Psychiatry. 2009;66:642–8. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crum WR, Griffin LD, Hill DL, et al. Zen and the art of medical image registration: correspondence, homology, and quality. Neuroimage. 2003;20:1425–37. doi: 10.1016/j.neuroimage.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 53.O’Donnell ML, Creamer M, Pattison P. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 2004;161:1390–6. doi: 10.1176/appi.ajp.161.8.1390. [DOI] [PubMed] [Google Scholar]
- 54.Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–9. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 55.Levy BJ, Anderson MC. Individual differences in the suppression of unwanted memories: the executive deficit hypothesis. Acta Psychol (Amst) 2008;127:623–35. doi: 10.1016/j.actpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 57.Moradi AR, Herlihy J, Yasseri G, et al. Specificity of episodic and semantic aspects of autobiographical memory in relation to symptoms of posttraumatic stress disorder (PTSD) Acta Psychol (Amst) 2008;127:645–53. doi: 10.1016/j.actpsy.2007.11.001. [DOI] [PubMed] [Google Scholar]