Fig. 1.
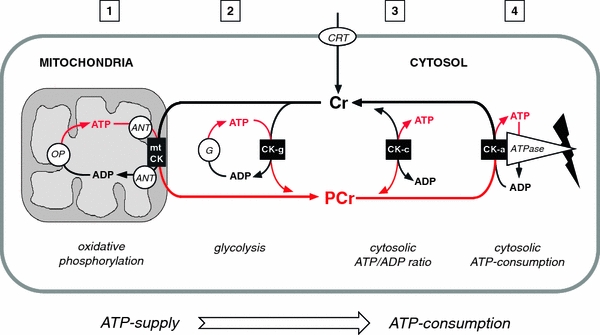
The CK/PCr system for temporal and spatial energy buffering in cells of high and fluctuating energy requirements. Cr enters the target cells via Cr transporter (CRT). Inside the cell, PCr/Cr and ATP/ADP equilibria are adjusted by a soluble fraction of cytosolic CK isoforms (CK-c, see (3)). Another fraction of cytosolic CK (CK-g, see (2)) is specifically coupled to glycolytic enzymes (G), accepting glycolytic ATP, while mitochondrial CK isoforms (mtCK, see (1)) is coupled to adenine nucleotide translocator (ANT), thus accepting ATP exported from the matrix and generated by oxidative phosphorylation (OP). The contribution of both of these so-called microcompartments to total PCr generation depends on the cell type. The PCr thus generated is fed into the large PCr pool (up to 30 mM) that is available as a temporal or spatial energy buffer. Another fraction of cytosolic CK (CK-a, see (4)) specifically associated with subcellular sites of ATP utilization (ATPase, e.g. ATP-dependent or ATP-gated processes, ion-pumps etc.) also forms tightly coupled microcompartments regenerating the ATP utilized by the ATPase reaction in situ on the expense of PCr. The proposed CK/PCr energy shuttle or circuit connects, via highly diffusible PCr and Cr, subcellular sites of ATP production (glycolysis and mitochondrial oxidative phosphorylation) with subcellular sites of ATP utilization (ATPases). This model is based on functionally coupled, subcellular CK microcompartments, where ATP production and ATP consumption are tightly connected to CK/PCr action (Wallimann 1975; Wallimann and Eppenberger 1985; Schlattner et al. 2006a; Wallimann et al. 2007)
