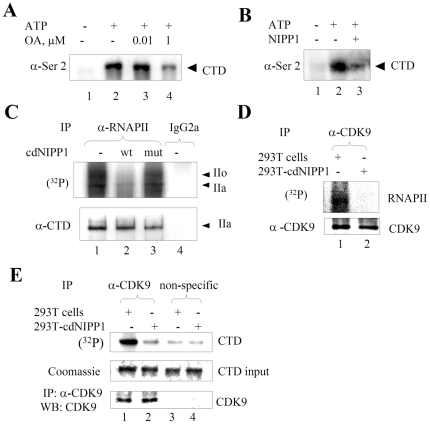
Figure 1. Inhibition of PP1 prevents RNAPII CTD phosphorylation and inhibits CDK9 activity.
(A) High concentration of okadaic acid inhibits RNAPII phosphorylation in vitro . HeLa cell nuclear extract was subjected to in vitro transcription without (lane 2) or with the addition of 10 nM okadaic acid (lane 3) or 1 µM okadaic acid (lane 4). Lane 1, untreated HeLa cell nuclear extract. RNAPII was resolved on 5% SDS-PAGE and analyzed with RNAPII CTD serine 2 phospho-epitope specific antibodies (Ser2). (B) NIPP1 prevents RNAPII phosphorylation in vitro. HeLa cell nuclear extract was subjected to in vitro transcription without (lane 2) or with the addition of 5 µM NIPP1 (lane 3). Lane 1, untreated HeLa cell nuclear extract. RNAPII was resolved on 5% SDS-PAGE and analyzed with Ser2 phospho-epitope specific antibodies. (C) Expression of cdNIPP1 prevents RNAPII phosphorylation in cultured cells. 293T cells were transfected with vectors expressing wt cdNIPP1 (lane 2) or mutant cdNIPP1 (lane 3) or mock transfected (lane 1). At 48 hours post transfection, the cells were treated with 0.1 µM okadaic acid and pulsed with (32P) orthophosphate for 3 hours. The cellular lysates were subjected to immunoprecipitation with 8WG16 antibodies against RNAPII CTD (lanes 1 to 3) or with non-specific mouse IgG2a (lane 4). Immunoprecipitated RNAPII was resolved on 5% SDS-PAGE and the gel was analyzed on Phosphor Imager. Separately, RNAPII was immunoprecipitated and analyzed by Western blotting (lower panel). (D & E) Expression of cdNIPP1 inhibits enzymatic activity of CDK9. Lysates of 293T cells (lane 1) or 293T cells continuously expressing cdNIPP1 (293T-cdNIPP1 cells) (lane 2) were immunoprecipitated with anti-CDK9 antibodies. Precipitated CDK9 was supplemented with γ-(32P) ATP and purified yeast RNAPII (panel D) or GST-CTD (panel E) as substrates. GST-CTD and RNAPII were resolved on 10% and 7.5% SDS-PAGE gels and the gels were analyzed on Phosphor Imager. Immunoprecipitation of CDK9 was verified by immunoblotting (lower panel D). Also there was phosphorylation in the absence of substrate ((lower panel E) or when non-specific antibodies were used (panel E, lanes 3 and 4). Results are from a typical experiment of 2–4 performed.