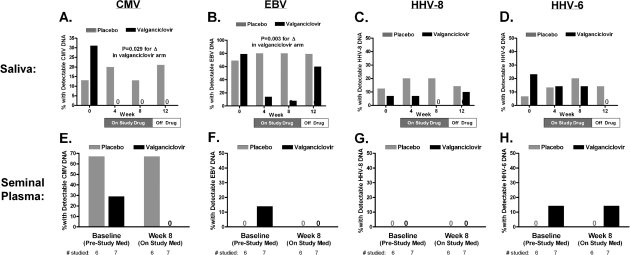
Figure 2.
Changes in herpesvirus DNA levels in saliva and semen with valganciclovir therapy. Herpesvirus DNA levels were assessed by polymerase chain reaction (lower limit of detection, 150 copies/mL) on saliva (at weeks 0, 4, 8, and 12) and seminal plasma (at weeks 0 and 8 only). The proportion of participants with a positive herpesvirus DNA level result is plotted for both valganciclovir and placebo groups in saliva and seminal plasma for cytomegalovirus (CMV) (A and E), Epstein-Barr virus (EBV) (B and F), human herpesvirus (HHV) type 8 (C and G), and HHV type 6 (D and H). Plotted P values test whether the proportion of participants with a positive DNA level result changes over time within each treatment group (Cochran Q test). The proportion of participants with positive CMV and EBV DNA levels in saliva declined significantly with valganciclovir therapy but not with placebo. The proportion of participants with detectable salivary CMV DNA levels remained suppressed 4 weeks after study drug discontinuation, whereas the proportion with detectable EBV levels rebounded to pretreatment levels. Whereas 2 (29%) of 7 valganciclovir-treated participants had detectable CMV DNA plasma at baseline, none had it at week 8.