Abstract
In many adult tissues, mesenchymal stem cells (MSCs) are closely associated with perivascular niches and coexpress many markers in common with pericytes. The ability of pericytes to act as MSCs, however, remains controversial. By using genetic lineage tracing, we show that some pericytes differentiate into specialized tooth mesenchyme-derived cells—odontoblasts—during tooth growth and in response to damage in vivo. As the pericyte-derived mesenchymal cell contribution to odontoblast differentiation does not account for all cell differentiation, we identify an additional source of cells with MSC-like properties that are stimulated to migrate toward areas of tissue damage and differentiate into odontoblasts. Thus, although pericytes are capable of acting as a source of MSCs and differentiating into cells of mesenchymal origin, they do so alongside other MSCs of a nonpericyte origin. This study identifies a dual origin of MSCs in a single tissue and suggests that the pericyte contribution to MSC-derived mesenchymal cells in any given tissue is variable and possibly dependent on the extent of the vascularity.
Keywords: dental pulp, tooth development, dental stem cells
The existence of mesenchymal stem cell (MSC) populations in adult tissues and organs that contribute to tissue cell turnover and also respond to tissue damage is a generally accepted concept. Adult stem cell niches potentially offer a source of autologous cells that could be used in a wide variety of stem cell-based treatments and therapies currently under investigation. However, their precise locations are often unclear and they are present in small numbers. The archetypal MSC population resides in bone marrow (1–3), but cells with similar molecular, phenotypic, and functional properties, at least in vitro, have been identified in a wide range of tissues, including adipose (4), dermis (5), and teeth (6–9). In addition to their common ability to differentiate into multiple mesenchymal cell types in vitro, these cells also share the common property of exhibiting immune suppressive activity (10, 11). The widespread nature of MSCs in adult tissues has led to suggestions that they have a common origin. Paradoxically, however, although they share common properties, they do display significant differences, such as the extent to which they form different cell types (12–15). In this context, recent attention has returned to pericytes/perivascular zones as candidates as a result of their broad distribution in all organs (16–22). Pericytes are an elusive cell type recognized by their anatomy and position rather than by a precisely defined phenotype. They reside on the abluminal surface of endothelial cells in the microvasculature of every vascularized connective tissue and have been postulated as the in situ counterpart of the bone marrow colony-forming units first described in the 1970s (1).
The potential for pericytes to contribute to the formation of tissues in addition to vessels has been established by numerous studies in several different tissues, including osteoblasts, chondroblasts, fibroblasts, adipocytes (16, 17), myogenic cells (18), and odontoblasts (19, 20). In addition, perivascular cells from multiple organs, including skeletal muscle, pancreas, adipose tissue, and placenta, were recently isolated based on the expression of CD146, NG2, and PDGF-Rβ, and showed that, after long-term culture, they retained myogenicity and exhibited, at the clonal level, osteogenic, chondrogenic, and adipogenic potentials, suggesting that the blood vessel walls harbor a reserve of progenitor cells that may be related to MSCs (18, 21, 22).
MSCs are largely defined retrospectively based on in vitro properties such as the capacity to proliferate extensively, form colonies, adhere to tissue culture plastic, and differentiate into mature mesenchymal lineages when induced with appropriate culture conditions. When these cells are transplanted into live animals, they are also usually subjected to in vitro expansion before transplantation. In vitro culture is variable and unable to mimic the stem cell niche, and moreover, somatic cells with a mature phenotype in vivo are able to dedifferentiate (23) or differentiate in other cell types in culture (24). Consequently, it is recognized that some of the MSCs defined operationally following in vitro culture may represent experimental artifacts (22).
To date, however, genetic-based linage tracing of pericyte differentiation has not been reported to definitively show a differentiated mesenchymal cell that originated from a pericyte. We chose to address this issue by using a physically and genetically accessible postnatal organ that shows continuous growth and self-repair—the mouse incisor. Rodent incisors continuously sharpen themselves by the shearing action of their tips. As tissue is lost during sharpening, this must be continuously replaced, and stem cells at the cervical end of the tooth have been identified that provide sources of new specialized cells (25). In common with other teeth, including human teeth, rodent teeth possess a limited repair response to damage of the dentine hard tissue and underlying specialized mesenchymal-derived dentine-producing cells—odontoblasts—mediated by MSC-like cells residing in the tooth pulp differentiating into new odontoblasts (26). We show that, during both incisor growth and repair following damage, odontoblasts can be identified that differentiate from pericytes. However, not all odontoblasts are pericyte-derived, and we identify a population of MSC-like cells that exhibit directed cell migration toward tissue damage and differentiate into odontoblasts. Although pericytes can differentiate in specialized mesenchymal cells, they do so alongside other MSC-like cells of nonpericyte origin.
Results and Discussion
Lineage Tracing of Pericyte Differentiation During Tooth Growth and Repair.
The NG2 is a proteoglycan that is commonly used as a marker for a gene expressed in pericytes (27). To use Cre-mediated genetic lineage tracing of pericytes, we used two different Cre-expressing mouse lines: a noninductive NG2cre and a tamoxifen-inducible NG2creER crossed with reporter lines (28–31). We first used NG2cre to establish the extent that Cre was expressed during odontoblast differentiation and then confirmed that the inducible Cre expression reproduced known sites of NG2 expression, including pericytes, following tamoxifen administration (Figs. S1 and S2). We also estimated the frequency of recombination from both Cre drivers by comparing LacZ expression with NG2 protein localization (30, 31) (Fig. S2).
During incisor growth, a small but reproducible number of positive cells were visible in the odontoblast cell layer, with their processes extending into the dentine of NG2creER;Rosa26R (Fig. 1C) and NG2cre;Z/EG mice (Fig. S2). Considering that, in the 4 d following tamoxifen treatment, only newly formed odontoblasts could be labeled, whereas in the NG2cre mice, all odontoblasts could have been labeled, we counted the number of LacZ-positive odontoblasts from the first positive cell visible in NG2creER;Rosa26R compared with the equivalent region in NG2cre;Z/EG. In NG2creER mice, 4 d after tamoxifen administration, a maximum of four or five odontoblasts were labeled in any one section, and in NG2cre mice, this figure was double. This corresponds to approximately 3% of odontoblasts labeled in NG2creER and 6% in NG2cre, and thus showed there were twice as many odontoblasts identifiable as being derived from pericytes in NG2cre than in NG2creER mice. This figure is comparable with the figure for the frequency of recombination following tamoxifen (50%), and suggests that, during normal incisor growth, as many as approximately 12% of odontoblasts are formed from pericytes. This pericyte contribution to odontoblast differentiation is not observed in noncontinuously growing teeth and is thus likely to occur as a consequence of the proximity of the large capillary plexus to the MSC niche in incisors.
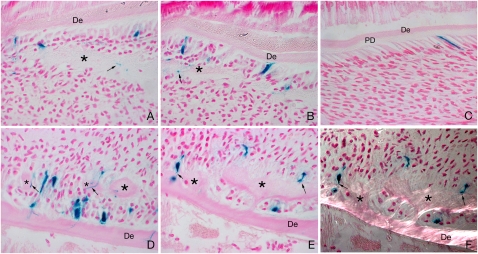
Fig. 1.
Pericytes can differentiate into odontoblasts following damage. NG2creER;Rosa26R pups were given one intraperitoneal injection of tamoxifen at P2 (4 mg/30 g body weight), and at 54 h after tamoxifen injection, mandibles on the left side were damaged by using a needle and the pups were culled 2 d (A and B) and 4 d (D and E) later. Right sides were used as controls (no damage) (C). After 2 d, the site of damage showed an eosinophilic cell matrix compatible with immature predentin (marked with asterisk) with close contact with NG2 LacZ-positive (NG2-positive) elongated odontoblast-like cells (A and B). At 4 d after damage, the number of LacZ-positive odontoblasts in close contact with the lesion greatly increased, showing clear signs of further differentiation with elongated bodies and long processes inside the reparative dentine (asterisk) canaliculus (D and E). Polarized light microscopy showed collagen fibrils in the reparative areas (asterisk), parallel to the odontoblast longitudinal axis (F). Black arrows show examples of LacZ-positive odontoblasts extending stubby processes inside the dentin matrix. De, dentin; PD, predentin.
Two days following experimental damage to the odontoblast cell layer, an increased number of positive cells were visible in the vicinity of the damage, which included cells with the characteristic polarized/rectangular shape of odontoblasts. At this time, there was no evidence of reparative dentine formation, but a cell matrix was visible in association with the positive cells (Fig. 1 A and B). Four days after damage, the number of positive cells had increased and now included polarized/rectangular cells with characteristic stubby processes extending into newly formed dentine-like tissue, a characteristic of odontoblasts (Fig. 1 D and E). The newly formed (reparative) dentine was recognizable by collagen fibrils arranged in the same plane as the odontoblasts. Formation of reparative dentine by NG2-positive odontoblasts was evident from their stubby processes extending into the collagen fibrils (Fig. 1 E and F). NG2-positive cells were thus able to differentiate into odontoblast-like cells and synthesize a reparative dentine-like tissue in response to damage.
Pericytes are thus clearly able to differentiate into specialized mesenchymal cells—odontoblasts—during growth and in response to odontoblast damage. However, in both these situations, they do not account for all the cell differentiation. Of the recognizable new odontoblasts associated with reparative dentine after 4 d, 15% were pericyte-derived. The small number of pericyte-derived odontoblasts seen during normal growth of the incisor suggests that another cell source, presumably cells from an incisor MSC-niche close to the cervical loop, contribute the majority of odontoblasts. In response to damage, the number of odontoblasts derived from pericytes is greater than during growth, but still does not account for all the cells, again suggesting mobilization of another source of MSCs of nonpericyte origin.
Pericyte Proliferation in Response to Tissue Damage.
Because the numbers of NG2-positive pericytes were very low in the resting pulp (Fig. S1 A–C), we investigated the mechanism that leads to the rapid increase in numbers at the site of tissue damage. We made use of a transgenic mouse, X-LacZ4 (aP2lacZ), which have nucleus β-gal expression in vascular smooth muscle cells and pericytes (32), including pericytes inside dental pulp mesenchyme (Fig. S3). In these mice, approximately two thirds of Ng2-expressing cells coexpress LacZ (Fig. S3).
As observed in the Ng2creER;Rosa26R incisors, very few positive cells were visible in the pulp except those associated with the blood vessel plexus located at the cervical end (Fig. 2A′ and B′). In vitro culture of these incisors accompanied by tissue damage showed a large increase in pericyte numbers compared with undamaged controls (Fig. 2 A and B). Because these incisors were cultured in vitro, the increase in pericyte numbers could not have come from any increase in the vasculature that accompanies an inflammatory response. Phospho-histone H3 (PH3) immunostaining showed that LacZ-positive cells close to the site of in vivo damage were proliferating (Fig. 2 C and C′). We thus conclude that damage to the odontoblast cell layer stimulates the proliferation of the few isolated pericytes resident in the tooth pulp.
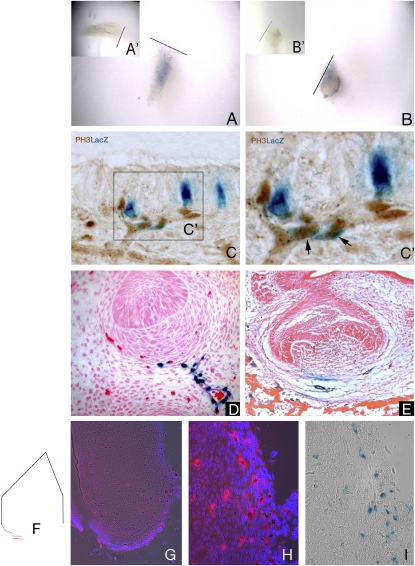
Fig. 2.
Pericytes can respond to injury locally, are involved in tooth development, and are slow-cycling cells. By culturing the X-LacZ4 incisors following damage, and thus eliminating the circulatory system, an increase in the number of LacZ-positive pericytes was evident in both pulp body (A) and cervical loop parts (B), compared with the control incisors (A′ and B′). These cells were characteristically dispersed throughout the pulp tissue, losing their organization within the main body plexus in the pulp body (A and A′), and were intensely concentrated on the bottom edge of the cervical loop area (B and B′), where a vessel-rich area is present. Coexpression of proliferation marker PH3 and LacZ-positive (NG2-positive) pericytes following in vivo damage (C and C′) showed that proliferating pericytes (black arrows) are located on blood vessels extending toward the damaged area (C′). During tooth development (bud to bell stages) in X-LacZ4 mice, LacZ-positive cells are found under the condensing mesenchyme (D) and in the blood vessels close to the forming tooth (E). When colocation of migrating slow-cycling cells and pericytes were analyzed by transplantation experiments associated with nucleoside (i.e., IdU) administration, some cells that migrated from the X-LacZ4 host were both LacZ-positive (pericytes, black stained cells) and IdU positive (slow-cycling, red stained cells) (G and H). These cells were found in the blood vessel-rich cervical loop area of incisors (F and G). Consecutive sections showed that these cells were rare but clustered (G), suggesting a spatial organization. High magnification showed colocalization of IdU and LacZ-positive pericytes under fluorescence (H) and bright field (I).
We next investigated the developmental origins of these small numbers of isolated pericytes in tooth pulp in X-LacZ4 (aP2lacZ) transgenic embryos. Positive cells were first detected at the late bud stage of tooth development [embryonic day (E) 13.5–14], when vessels first appear. Pericytes were seen around capillaries close to the developing teeth and also as a stream with the appearance of cells migrating from the vessel wall into the mesenchymal cells condensing around the epithelial tooth bud (Fig. 2D). By the cap stage of tooth development (E14.5–15), pericytes could be seen on vessels outside the tooth primordium and as a few scattered cells in the mesenchymal cells of the dental papilla, the cells that will form the pulp (Fig. 2E). Pericytes thus infiltrate the mesenchymal cell population destined to form the tooth pulp early in development. The approximate numbers of pericytes seen in sections of the cap stage tooth primordia was consistently greater than the numbers observed in sections of adult teeth, suggesting that these pericytes had not increased significantly in number from those infiltrating the mesenchyme during development. To confirm that tooth pulp pericytes are slow cycling, nucleoside analogue incorporation assays were performed. Administration of the nucleoside IdU in the drinking water of adult X-LacZ4 males for 4 wk followed by a chase for 20 d was expected to label only the slowest-cycling cells. C57BL6 E10.5 or E14.5 first branchial arch primordia were transplanted under kidney capsules of X-LacZ4 males. After 2 or 3 wk, the kidneys were removed, the teeth dissected, and immunofluorescence for IdU and LacZ staining used to identify correspondingly slow-cycling cells and pericytes. Sections of tooth pulp identified IdU -positive/LacZ -positive cells that were slow-cycling pericytes that had migrated into the pulp via the host vasculature (Fig. 2 G–I).
A small number of pericytes not associated with vessels populate the tooth mesenchyme during development, where they remain essentially quiescent until damage to the odontoblast cell layer occurs, as a result of extensive caries, for example. Odontoblast damage stimulates the proliferation of pericytes that are then able to contribute to the formation of new odontoblasts.
Mesenchymal Stem Cell Niche Close to Cervical Loop Responds to Odontoblast Damage.
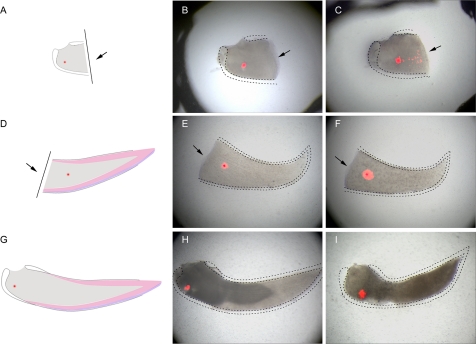
Because, during natural incisor growth and in damage repair, only a small percentage of newly differentiating odontoblasts were derived from pericytes, there must be another source of MSCs in the incisor pulp. The location of the MSC niche in mouse incisors has not been described, but it is assumed to be located close to the cervical end, where odontoblast differentiation begins. We therefore used 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) labeling of mesenchymal cells in different regions of the incisor pulp to identify any responses to tooth damage. Labeling of mesenchymal cells approximately 400 μm from the cervical opening of the incisor, followed by damage more distally, revealed directed migration of cells toward the damage after 2 d (Fig. 3 A–C). In the absence of damage, no directed cell migration was observed and cells labeled in other areas showed no migration toward the area of damage (Fig. 3 D–I). This identifies a discrete population of mesenchymal cells at the cervical end of the incisor that undergo directed cell migration (cell homing) following tooth damage.
Fig. 3.
Directed cell migration toward tissue damage visualized by DiI labeling of incisor mesenchyme pulp cells. Cells labelled in cervical loop area (A–C), damaged pulp cells area (A–C), damaged pulp body (D–F), and control teeth with no damage (G–I). Arrows indicate position of tooth damage. A lesion separating the cervical loop and the pulp body was provoked in incisors, and, 48 h later, mesenchymal cells were capable of migrating toward the site of the damage (A–C) whereas the mesenchymal cells from the pulp body showed no migration (D–F). In the absence of the lesion, no directed migration of cervical loop mesenchymal cells was observed (G–I).
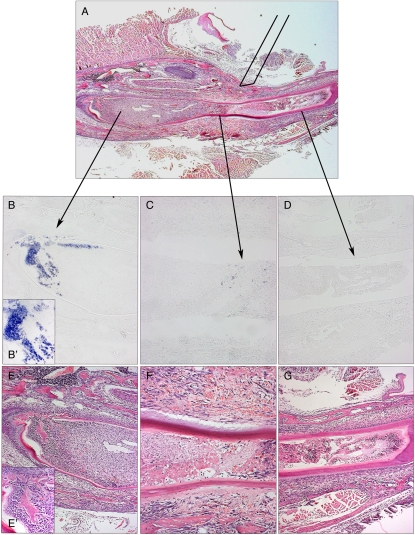
If this cell homing is part of the natural repair process, we reasoned that these cells should differentiate into odontoblasts. As the incisors did not survive in in vitro culture long enough for us to detect new odontoblasts from their morphology, we used expression of the dentinsialophosphoprotein (Dspp) gene to detect early odontoblast differentiation (33, 34). An incisor was damaged in three different places in vivo: directly into the area close to the cervical loop, midway along its length of the tooth, and close to the distal tip and cultured for 4 d (Fig. 4A). In situ hybridization for Dspp expression showed a massive induction in cells at the cervical loop end (Fig. 4 B and B′). Midway along the tooth, a few individual Dspp-expressing cells were visible (Fig. 4C), whereas at the tip, there were no positive cells (Fig. 4D). Thus, odontoblast differentiation is stimulated by damage, and the extent of differentiation at a given period after the damage correlates with the distance from the area from which cells migrate. The contribution of cells differentiating into new odontoblasts following damage is thus from at least two different sources: pericytes resident as quiescent cells in the pulp whose proliferation is stimulated by the damage, and migration of cells from the MSC niche area of the tooth that normally provide a continuous cell supply to support tooth growth. These represent two distinct cell populations that contribute to the formation of most of the odontoblasts in growth and repair.
Fig. 4.
Differentiation into Dspp-positive preodontoblasts following damage. Following damage to three separate sites, Dspp expression was visualized by in situ hybridization to reveal early odontoblast differentiation. Damage directly to the cervical loop area showed extensive expression of Dspp after 2 d (B and B′), whereas only a few expressing cells were visible in the damaged region midway along the length of the tooth (C). At the tip of the tooth, no Dspp expression was evident (D). Note that evidence of an inflammatory response can be seen in the tip (G), body (F), and cervical loop cells (E), but presence of osteodentin is evident in only the cervical loop (E′).
Concluding Remarks.
The extent to which perivascular cells act as MSCs during tissue growth and repair has long been a contentious issue. Although a somewhat circular argument, the fact that many MSCs in vivo and in vitro express genes also expressed by pericytes is often used to claim a pericyte origin. Similarly, isolation of perivascular cells from tissues, based on expression of these markers and retention of MSC-like properties followed by long-term culture, has provided compelling evidence that pericytes can act as a generic source of MSCs (18, 35). What the current evidence has lacked is a demonstration of genetically marked pericytes differentiating into mesenchymal cells in vivo. Our results provide this evidence, at least for a tissue during growth and repair, but show that pericytes are not the only cell lineage that acts as a source of MSCs.
We suggest that the relative contribution of pericyte-derived and non–pericyte-derived MSCs to cell differentiation in any given tissue depends on the extent of the vascularity and the kinetics of growth and/or repair. This can explain the conflicting data on the pericyte contribution to MSC-derived cells in different tissues. Thus, in tissues with low vascularity, such as articular cartilage, the pericyte contribution to MSCs will be less than in tissues with more extensive blood supplies.
An obvious question this raises is why are there two mechanisms (cell sources) for mesenchymal tissue repair. One hypothesis is that this is an evolutionary adaptation to facilitate rapid tissue repair whereby stem cells can quickly accumulate at a site of damage via the inflammatory response (36).
Materials and Methods
Mouse Lines.
The transgenic X-LacZ4 mouse line contains a single copy of the transgene integrated into the host genome. At late developmental stages and in the adult, LacZ staining marks vascular smooth muscle cells throughout the vascular bed, with the exception of the major elastic arteries, and in pericytes (32). The NG2cre mouse line consists of NG2cre BAC transgenic mice expressing Cre under the control of the NG2 promoter [Cspg4 (ng2) gene] (30). Inducible NG2CreERBAC transgenic mice were generated by using the same approach except that NG2cre cDNA was replaced by NG2creER cDNA (28). NG2cre and NG2creER were mated with Z/EG (29) and Rosa26R reporter mice (31), respectively, to detect cre-mediated recombination. Postnatal day 2 (P2) pups were given a single intraperitonial injection (4 mg/30 g body weight) of tamoxifen (T5648; Sigma) dissolved in corn oil (C-8247; Sigma). fifty-four hours after the tamoxifen injection, the mandibles at the left side were damaged using an 18-gauge needle and the pups were culled 2 and 4 d following damage, respectively. Right sides were used as controls. NG2cre;Z/EG pups of similar age (P1) were culled for comparative analysis of overall NG2cre activity during tooth growth. The number of LacZ-positive odontoblasts in the injured area were counted on a total of 12 sections from three incisors.
LacZ Expression.
Samples were fixed in 1% paraformaldehyde (PFA), 0.2% glutaraldehyde, in PBS solution for a time depending on the size of the sample followed by 48 h of staining at 37 °C for detection of β-gal activity using a solution composed of: 10 mM Tris-HCL pH 7.3, 0.005% Na-dexoxycholate, 0.01% IGEPAL (Sigma), 5 mM K4Fe (CN)6, 5 mM K3Fe(CN)6, 2 mM MgCl2, and 0.8 mg/mL X-Gal. As positive and negative controls, ROSA26 and CD1 mouse lines were used, respectively.
Recombination Efficiency.
The number of LacZ-positive odontoblasts in NG2cre;Z/EG and NG2creER;Rosa26R incisors were counted in sagittal sections. As NG2cre is active for a longer period than NG2creER, 4 d after tamoxifen administration, we compared the number of LacZ-positive cells per an equivalent number of odontoblasts (n = 50) in a region proximal to the last forming labeled cell. Cells were counted on a total of 14 sections from three incisors.
Response of Pericytes Following Incisor Damage in Culture.
Lower incisors at p2 from the X-LacZ4 mouse line were cultured for 5 d. The incisors were removed from the jaws and dissected, and the outer epithelium and developing enamel were physically removed. The cervical loop and the pulp body were separated from each other using a blade to cause pulp and odontoblast damage. The separated tooth parts were cultured on Millipore 0.10-μm filters exposed to oxygen. Medium (DMEM 10%, FBS 1%, penicillin/streptomycin solution) was changed every day. After 5 d, culture samples were photographed, fixed, stained for detection of β-gal activity, and embedded for histological analysis.
Immunohistochemistry.
Immunohistochemistry was performed on 5- to 7-μm paraffin sections of samples with or without LacZ staining. Heat-based antigen retrieval was performed before primary antibody incubation. The primary antibodies anti-NG2 chondroitin sulfate proteoglycan (AB5320; Millipore), anti–phospho-histone H3 (Ser10) (06-530; Millipore), α-smooth muscle actin (α-SMA; ab5694; Abcam), and GFP (ab6556, Abcam) were used at 1:50, 1:100, 1:500 and 1:500 dilution, respectively. Vectastain Elite ABC Kit (rabbit IgG) (PK-6101; Vector Labs) and DAB Peroxidase Substrate Kit (SK-4100; Vector Labs) were then used according to manufacturers’ instructions. Hematoxylin counterstain (03971; Fluka) was performed only on non–LacZ-stained samples to avoid masking the LacZ signal.
Colocation of Migrating Pericytes and Slow-Cycling Cells.
Seven 6-wk-old X-LacZ4 males received the nucleoside iododeoxyuridine (IdU) in drinking water (1 mg/mL) for 4 wk. After a washout period of 20 d, C57BL6 mandible primordia at different ages (E10.5–E14.5) were transplanted under kidney capsules of adult male mice. After 2 or 3 wk (depending on the age of the transplanted mandible), the tissue was removed from the kidneys, the mandibles dissected, and IdU immunofluorescence for IdU and LacZ staining used to identify slow-cycling cells and pericytes, respectively. Both slow-cycling cells and pericytes would always be originally from the host, and cells coexpressing both markers would be slow-cycling-migrating pericytes. For IdU staining, primary antibody was IdU (1:10, ab8152; Abcam) and secondary antibody was anti–mouse-cy3 (1:300, 87173; Jackson ImmunoResearch Laboratories), tissue was fixed in 4% PFA, decalcified (10% Na citrate, 10% formic acid, and 1% PFA in PBS solution), and paraffin-embedded. Consecutive sections of whole teeth were analyzed.
Migration Capacity Following Incisor Damage in Culture.
Lower incisors at p2 from CD1 mice were cultured for 2 d. The incisors were removed from the jaws and dissected, and the outer epithelium and developing enamel physically removed. The cervical loop and the pulp body were separated from each other by using a blade. Incisor bodies and cervical loops were labeled with 1 mg/mL of CM-DiI (C-7000; Molecular Probes). The separated tooth parts were cultured on 0.4-μm cell culture membranes (35–3090; Becton Dickinson) exposed to oxygen. Medium (DMEM 10%, FBS 1%, penicillin/streptomycin Fungizone solution) was changed every day, and samples were photographed and after 2 d.
Mobilization Capacity After Injury.
Lower incisors of CD1 mice at P5 were damaged with an 18-gauge needle that was pressed against the tissue, resulting in a pierced area and/or tissue dislodgement. After 2 d, the animals were euthanized, and jaws were dissected, fixed, and analyzed histologically. Sequential slides were submitted to Dspp digoxigenin-labeled in situ hybridization on sections as previously described (37).
Supplementary Material
Acknowledgments
We thank Dirk Dietrich (University of Bonn) for NG2creER mice and Moshe Shani (Volcani Center, Israel) for permission to use the X-LacZ4 mice. We thank Alex Huhn for his invaluable technical assistance. This work was supported by MRC Grant G0600041 (to P.T.S.). C.D.B. is a Medical Research Council Fellow (G108/620).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015449108/-/DCSupplemental.
References
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toma JG, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 6.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronthos S, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 8.Miura M, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonoyama W, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 11.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 12.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 13.Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegyi B, et al. Identical, similar or different? Learning about immunomodulatory function of mesenchymal stem cells isolated from various mouse tissues: Bone marrow, spleen, thymus and aorta wall. Int Immunol. 2010;22:551–559. doi: 10.1093/intimm/dxq039. [DOI] [PubMed] [Google Scholar]
- 16.Nehls V, Drenckhahn D. The versatility of microvascular pericytes: From mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- 17.Lin G, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Alliot-Licht B, Hurtrel D, Gregoire M. Characterization of alpha-smooth muscle actin positive cells in mineralized human dental pulp cultures. Arch Oral Biol. 2001;46:221–228. doi: 10.1016/s0003-9969(00)00115-1. [DOI] [PubMed] [Google Scholar]
- 20.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 21.Satokata I, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Romero J, et al. Immunophenotypic analysis of human articular chondrocytes: Changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 24.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 25.Harada H, et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AJ, et al. Reactionary dentinogenesis. Int J Dev Biol. 1995;39:273–280. [PubMed] [Google Scholar]
- 27.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 31.Novak A, Guo CY, Yang WY, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 32.Tidhar A, et al. A novel transgenic marker for migrating limb muscle precursors and for vascular smooth muscle cells. Dev Dyn. 2001;220:60–73. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1089>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Feng JQ, et al. Genomic organization, chromosomal mapping, and promoter analysis of the mouse dentin sialophosphoprotein (Dspp) gene, which codes for both dentin sialoprotein and dentin phosphoprotein. J Biol Chem. 1998;273:9457–9464. doi: 10.1074/jbc.273.16.9457. [DOI] [PubMed] [Google Scholar]
- 34.Sreenath TL, Cho A, MacDougall M, Kulkarni AB. Spatial and temporal activity of the dentin sialophosphoprotein gene promoter: Differential regulation in odontoblasts and ameloblasts. Int J Dev Biol. 1999;43:509–516. [PubMed] [Google Scholar]
- 35.Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 36.Kurth TB, et al. Functional mesenchymal stem cell niches in the adult knee joint synovium in vivo. Arthritis Rheum. 2011 doi: 10.1002/art.30234. in press. [DOI] [PubMed] [Google Scholar]
- 37.Nakatomi M, Morita I, Eto K, Ota MS. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 2006;85:427–431. doi: 10.1177/154405910608500506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.