Abstract
Endothelial progenitor cells (EPCs) derived from bone marrow are known to be heterogeneous. In this study, we tried to find favorable conditions that induce the differentiation of mononuclear cells (MNCs) from bone marrow into EPCs. The differentiation capacity of MNCs from rat bone marrow was investigated in different conditions, such as different media, different induction times and different culture surfaces. The cell morphology and endothelial biomarkers associated with differentiated MNCs were studied. Our results indicated that MNCs cultured in EGM-2MV (Endothelial cell basal medium-2, plus SingleQuots of growth supplements) developed a bursiform shape, a late EPC-like morphology, while MNCs cultured in complete medium (CM, M199 with 10% FBS, 20 ng/mL VEGF and 10 ng/mL bFGF) showed a spindle shape, an early EPC-like morphology. Cells of both morphologies were able to incorporate DiI-ac-LDL and bind lectin in vitro. MNCs cultured in EGM-2MV exhibited a higher proliferation rate and higher eNOS expression than MNCs cultured in CM. MNCs cultured in EGM-2MV had the ability to form tubes on Matrigel. Flow cytometry results indicated that CD133 expression was highest at day 12 and that the greatest number of cells positive for both FLK-1 and CD133 appeared at day 20 from cells cultured in dishes without fibronectin coating. In addition, the expression levels of CD133, CD31 and FLK-1/CD133 were not significantly different between cells of different shapes. Our experiments suggest that MNCs from bone marrow can be differentiated into late EP-like cells in EGM-2MV, which have the ability to rapidly proliferate. These MNCs can also be differentiated into early EP-like cells in CM. Additionally, fibronectin may not be necessary for the differentiation of EPCs to mature ECs after three generations. Differentiated MNCs from bone marrow in EGM-2MV have the characteristics of EPCs, although the expression levels of EPC markers were lower than previously reported.
Keywords: Endothelial progenitor cells, Mononuclear cells, CD133, FLK-1
Introduction
In 1997, Asahara et al. (1997) reported that CD34-positive (CD34+)-enriched mononuclear cells from peripheral blood could be differentiated into EPCs with endothelial structure and function, which provided the first evidence that there were endothelial progenitors, the precursors known as hemangioblasts, in human peripheral blood. These EPCs could participate in prenatal vasculogenesis and angiogenesis after birth. The infusion of EPCs isolated from peripheral blood or bone marrow was shown to improve blood flow after ischemia and rescue endothelial injury (Werner et al. 2003). Because of the histoincompatibility of different individuals, it is beneficial to have many sources of EPCs. Several sources of EPCs currently exist, such as peripheral blood, bone marrow and tissues. Additionally, scientists are interested in bone marrow-derived cells as a therapeutic source of vascular cells.
At present, ex vivo expanded EPCs present many difficulties. There are no uniform biomarkers that can specifically identify the EPCs (Schatteman et al. 2007); thus, one source of cells can be conditionally differentiated into cells with different phenotypes. Because of the inability to specifically identify EPCs, EPCs cultured by different groups using different separation methods show different morphologies, surface markers and biologic functions. Asahara et al. (1997) found that CD34+ peripheral blood mononuclear cells (PBMCs) could differentiate into endothelial-like cells expressing CD31, Tie-2, CD34 and VEGFR2. Endothelial-like cells from CD14+ PBMCs expressed both monocytic antigens and EC antigens, and these cells could also uptake ac-LDL and bind Ulex (Gulati et al. 2003). Friedrich et al. found that a CD34−/CD133+ EPC subpopulation was a precursor of CD34+/AC133+ EPCs and contributed to vascular regeneration more potently than other subpopulations (Friedrich et al. 2006). Although CD34, CD133 and FLK-1 were previously used as positive surface markers of EPCs, Schatteman et al. (2007) suggested that other characteristics such as size, cell cycle, and other functions may be more useful to identify EPCs than cell surface markers alone. Ingram et al. (2005) used proliferative potential as one defining aspect of EPC biology to make a hierarchy of EPCs in human blood and blood vessels. Therefore, caution must be taken, and we should use as many different methods to define EPCs as possible, especially when classifying cells derived from bone marrow.
Currently, the isolation of EPCs is achieved by either magnetic-activated cell sorting (Asahara et al. 1997) or by a differential attachment method (Jin et al. 2004), in which cells are cultured on different matrices for different periods of time. Both isolation methods have proven to be effective. For the separation of rat bone marrow EPCs, the use of magnetic-activated cell sorting presents some difficulty because antibody selection is limited and because the biological characteristics of rat bone marrow EPCs are poorly defined. In this study, EPCs were separated from MNCs via the differential attachment method, and the function and biomarkers of EPCs were investigated in different culture conditions.
Materials and methods
Isolation of mononuclear cells from rat bone marrow
All animal procedures were approved by the laboratory animal ethics committee of Taishan Medical University and conformed to the national guidelines for care and use of laboratory animals. Isolation and culture of MNCs were described previously (Yin et al. 2010). Bones from 4-week-old SD male rats were repeatedly washed with PBS until the washing fluid of the bone marrow cavity became clear. The washing fluid was filtered into a single-cell suspension with a 100-μm mesh. Single-cell suspension was added to Ficoll at a ratio of 1:1. The mixture was then centrifuged at 2,000 rpm for 20 min at 20 °C to separate the cells into three layers. The middle layer, which is white and cloudy and consists of the mononuclear cells, was gently removed and transferred to a new centrifuge tube. The cells were washed two times with PBS, resuspended with complete medium (CM, M199 with 10% FBS, 20 ng/mL VEGF and 10 ng/mL bFGF) or EGM-2MV (Lonza, Endothelial cell basal medium-2, plus FBS, VEGF, R3-IGF-1, rhEGF, rhFGF-B, GA-1000, hydrocortisone and ascorbic acid) and seeded in fibronectin-coated plates or culture bottles to a cell density of 106/cm2. Four days later, non-adherent cells were washed off with PBS, and fresh media were added to the cultures every 3 days.
DiI-ac-LDL uptake and FITC-UEA binding assay
EPCs were incubated with 2.5 mg/L DiI-ac-LDL (Molecular Probes) for 2 h at 37 °C. The cells were then fixed for 5 min with 1–2% paraformaldehyde. The fixed cells were washed with PBS, incubated with 10 mg/L of FITC-UEA (Sigma) for 1 h at 37 °C and analyzed using a fluorescent microscope. Cells staining positively for both markers were considered to be differentiating EPCs, as reported previously (Ingram et al. 2004).
EPC adhesion and MTT proliferation assay
EPCs were seeded on fibronectin (BD)-coated plates after digestion and were cultured for 30 min in different media. Dishes were vigorously washed three times with changes of PBS, and adherent cells were counted under a microscope (magnified 200×). Next, 2,000 cells per well were seeded in fibronectin-coated 96-well plates for 24 h. After serum starvation for 24 h, 20 μL of 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT, 5 g/L) (Sigma) was added to each well, and the cells were cultured for 4 h at 37 °C. The supernatant was then removed, and 150 μL of dimethyl sulfoxide (DMSO, Gibco) was added to each well. After shaking for 10 min at room temperature, cell proliferation was assessed by measuring the absorbance at 492 nm with a microplate reader.
FCM detection of surface markers on EPCs
Adherent cells were digested into a single-cell suspension, blocked for 30 min and incubated with antibodies against rat CD133 (Abcam, from rabbit), rat FLK (Santa Cruz, from mouse) or rat CD31 (BD, from mouse) for 30 min at 4 °C. One tube was not incubated with any antibody as negative control. After washing twice with PBS, the cells were incubated with a FITC-goat anti-rabbit secondary antibody (BD) and an APC-labeled goat anti-mouse secondary antibody (BD) for 30 min at 4 °C, washed twice with PBS, and fixed with 4% paraformaldehyde. EPC surface markers were analyzed by flow cytometry (FCM).
Tube formation on matrigel
EPCs were seeded on matrigel-coated plates and cultured with EGM-2MV for 24 h at 37 °C, 5% CO2. Tube formation was monitored by microscope.
Immunofluorescence
Differentiated EPCs were analyzed for the markers of endothelial cells. Differentiated EPCs were incubated with anti rat CD133 (Abcam, from rabbit), rat FLK (Santa Cruz, from mouse) or rat CD31 (BD, from mouse) for 60 min at room temperature. Positive staining was detected using Cy3 or FITC conjugated secondary antibodies by fluorescent microscope.
Immunoblot analysis
Whole cell lysates (30 μg/lane) were electrophoresed in a 10% non-denaturing polyacrylamide gel, followed by electrophoretic transfer to PVDF membranes (Millipore), and incubated overnight with primary antibodies against eNOS (Santa Cruz). The membranes were then exposed to secondary antibodies conjugated with horseradish peroxidase (Santa Cruz) and detected by the Phototope-HRP Western Detection Kit (Thermo).
Statistical analysis
All data are presented as mean ± SEM. Intergroup comparisons were performed by paired Student t tests or ANOVA. Probability values of p < 0.05 were interpreted to denote statistical significance.
Results
The characteristics of rat bone marrow MNCs induced by different media
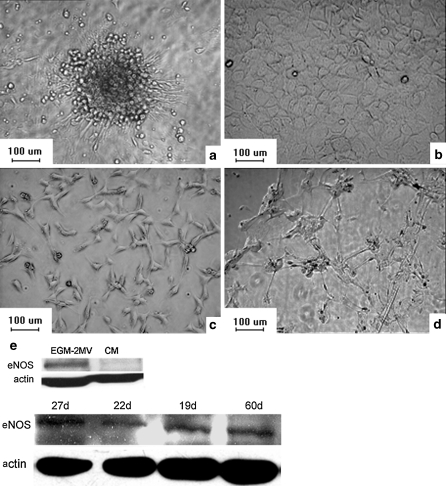
MNCs isolated from rat bone marrow were cultured in EGM-2MV or complete medium (CM, M199 with 10% FBS, 20 ng/mL VEGF and 10 ng/mL bFGF). After removing non-adherent cells at day 4, culture of the adherent cells continued in the corresponding media. The MNCs cultured in EGM-2MV proliferated more rapidly than the MNCs in CM during the first week of culture. The adherent cells in CM formed colonies surrounded by spindle-shaped cells, an early EPC phenotype as Asahara et al. first reported (Fig. 1a) (Asahara et al. 1997), and gradually differentiated to have a cobblestone-like morphology (Fig. 1b). In contrast, the MNCs cultured in EGM-2MV showed a fusiform shape (Fig. 1c) or a cobblestone shape, could be passaged for over 2 months without senescence and had the appearance of late EPCs (Lin et al. 2000), in that they could form capillary-like sprouts on Matrigel (Fig. 1d). Expression of eNOS in EPCs was lower in CM than in EGM-2MV and did not change over time (Fig. 1e).
Fig. 1.
The different phenotypes of the MNCs in different conditions. a Rat MNCs from bone marrow in CM formed colonies surrounded by spindle-shaped cells (magnified 10×). b Rat MNCs from bone marrow in CM differentiated into a cobblestone-like morphology (magnified 10×). c Rat MNCs from bone marrow in EGM-2MV formed a fusiform shape (magnified 10×). d EPCs formed capillary-like sprouts on Matrigel (magnified 10×). e The expression of eNOS in EPCs induced by different media for different culture times
The induced EPCs could incorporate ac-LDL and bind UEA-1 and differentiate into mature endothelial cells (ECs)
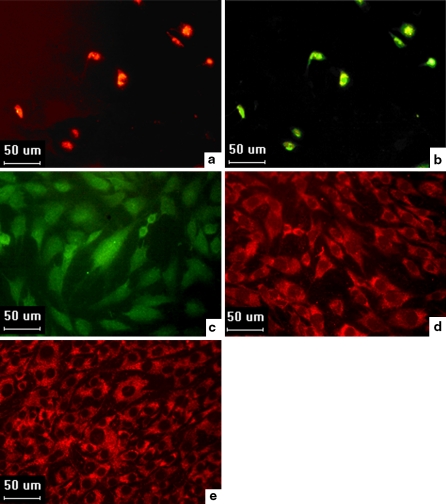
The differentiated MNCs derived from rat bone marrow, cultured in either EGM-2MV or in CM, demonstrated features of EPCs in that they were able to take up ac-LDL (Fig. 2a) and bind lectin (Fig. 2b) at day 11. Some studies, however, have indicated that Ulex-binding and ac-LDL uptake, which have previously been used to identify differentiating EPCs, are not specific for EPCs (Sandri et al. 2005). In order to further indentify EPCs, these cells induced by EGM-2MV for a longer time could differentiated into mature ECs with expression of makers, such as vWF, CD31 and FLK-1 (Fig. 2c, d, e).
Fig. 2.
The uptake of DiI-ac-LDL, binding of FITC-UEA-1, and surface markers expression by EPCs. a Uptake of DiI-ac-LDL by EPCs (magnified 40×). b Binding of FITC-UEA-1 by EPCs (magnified 40×). c,d,e surface markers expression by the differentiated EPCs: vWF (c), CD31 (d), and FLK-1 (e) (magnified 40×)
The proliferation and adhesion ability of induced rat bone marrow EPCs in different culture conditions
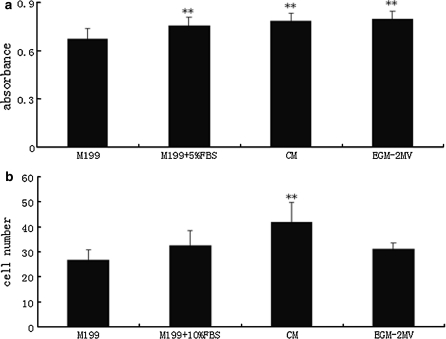
Rat bone marrow-derived EPCs cultured in CM for 14 days were passaged into different media to study their biological functions, such as proliferation and adhesion. EPCs cultured in EGM-2MV proliferated more rapidly than EPCs in other culture media. The serum significantly contributed to their proliferation capacity (p < 0.01, Fig. 3a). EPCs grown in CM demonstrated the strongest adhesion ability as they had the greatest number (41.9 ± 7.9) of adherent cells (p < 0.01, Fig. 3b).
Fig. 3.
MNCs induced by different culture conditions showed differences in biological properties. Data are presented as the mean ± SD n = 5 (All **p < 0.01 vs. M199 group). a The proliferative ability of MNCs in different culture media (y-axis: Absorbance at 492 nm—MTT proliferation assay). b The adherence ability of MNCs in different culture media (10000 cells were seeded on fibronectin-coated plates in different media. Cell number (y-axis) is the average of four fields which were counted under microscope (magnified 200x)).
Identification of EPC biomarkers in rat bone marrow-derived MNCs by flow cytometry
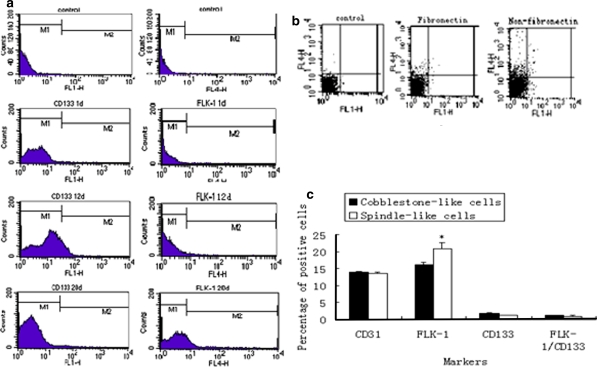
Rat bone marrow-derived MNCs were plated on fibronectin-coated dishes with EGM-2MV. After removing non-adherent cells on day 4, the adhering cells grew slowly for the first 7 days; therefore, there were not enough cells for us to examine cell surface markers by FCM. The cells were confluent on day 11, and we passaged them continuously. We investigated surface markers of these differentiated MNCs by FCM. We were able to detect the expression of CD133 (0.22 ± 0.072%), FLK-1 (0.30 ± 0.076%), and CD133/FLK-1 (0.047 ± 0.015%) on day 1, but by day 12, expression of each of these markers had increased (CD133 (9.51 ± 0.612%), FLK-1 (0.44 ± 0.079%), CD133/FLK-1(0.33 ± 0.068%)). FLK-1(18.91 ± 3.17%) and CD133/FLK-1(1.03 ± 0.16%) were most highly expressed on day 20 (Fig. 4a, Table 1).
Fig. 4.
Flow cytometric analysis of surface markers of EPCs differentiated from rat bone marrow MNCs. Data are presented as the mean ± SD, n = 3 (*p < 0.05, **p < 0.01). a The expression of EPC markers change over time in culture, corresponding percentage in Table 1. b Detection of EPC markers in rat bone marrow-derived MNCs plated on either fibronectin-coated dishes or non-fibronectin-coated dishes, corresponding percentage in Table 2. The expression of CD133 and FLK-1 were separately detected using FL-1H and FL-4H, respectively. Control represents negative control without primary antibody incubation. c The expression of biomarkers in cobblestone-shaped cells and spindle-shaped cells, corresponding percentage in Table 3
Table 1.
The percentage of EPCs markers with different culture time
| CD133 | FLK-1 | CD133/FLK-1 | |
|---|---|---|---|
| 1 day MNCs | 0.22 ± 0.072 | 0.30 ± 0.076 | 0.047 ± 0.015 |
| 12 days MNCs | 9.51 ± 0.612 | 0.44 ± 0.079 | 0.33 ± 0.068 |
| 20 days MNCs | 1.44 ± 0.43 | 18.91 ± 3.17 | 1.03 ± 0.16 |
In addition, we observed that fibronectin was necessary for the first three generations of EPC culture, but it had no significant impact on the proliferation of EPCs at later times. To further confirm that fibronectin did not contribute to the culture of EPCs after three passages, we plated the EPCs in both fibronectin-coated dishes and non-fibronectin-coated dishes and examined the expression of the EPCs’ biomarkers. The results showed that the percentages of cells that were plated on fibronectin-coated dishes were positive for CD133, FLK-1, CD31, and CD133/FLK-1 were 0.43 ± 0.035, 1.40 ± 0.191, 4.23 ± 0.515, and 0.23 ± 0.036%, respectively. The percentages of cells that were plated on non-fibronectin-coated dishes were positive for CD133, FLK-1, CD31, and CD133/FLK-1 were 0.56 ± 0.098, 4.27 ± 0.703, 2.40 ± 0.443, 0.35 ± 0.051%, respectively (Fig. 4b, Table 2).
Table 2.
The percentage of EPC markers in rat bone marrow-derived MNCs plated on either fibronectin-coated dishes or non-fibronectin-coated dishes
| CD133 | FLK-1 | CD133/FLK-1 | CD31 | |
|---|---|---|---|---|
| Fibronectin | 0.43 ± 0.035 | 1.40 ± 0.191 | 0.23 ± 0.036 | 4.23 ± 0.515 |
| Non-fibronectin | 0.56 ± 0.098 | 4.27 ± 0.703** | 0.35 ± 0.051* | 2.40 ± 0.443** |
All * p < 0.05, ** p < 0.01 versus fibronectin group
In the initial culture of MNCs, we found that there were two populations of cells in the fibronectin-coated dishes: one exhibited a cobblestone shape, and the other displayed a spindle-like shape. The cell populations were separated on day 11 and continuously cultured on fibronectin-coated dishes for 9 days. In addition to FLK-1, other biomarkers such as CD133, CD31, and CD133/FLK-1 were more highly expressed in the cobblestone-shaped cells than in the spindle-shaped cells (Fig. 4c, Table 3).
Table 3.
The percentage of EPC markers in rat bone marrow-derived cobblestone-shaped cells or spindle-shaped cells
| CD133 | FLK-1 | CD133/FLK-1 | CD31 | |
|---|---|---|---|---|
| Cobblestone-shaped cells | 1.82 ± 0.16 | 16.2 ± 0.79 | 1.16 ± 0.06 | 13.96 ± 0.2 |
| Spindle-shaped cells | 1.1 ± 0.09 | 21.04 ± 1.72* | 0.95 ± 0.12 | 13.64 ± 0.32 |
All * p < 0.05 versus Cobblestone-shaped cells
Discussion
Currently, there is no simple way to differentiate bone marrow-derived MNCs into abundant EPCs at equal pace, as MNCs derived from bone marrow are often dormant (Bradford et al. 1997) and comprise different cell populations (stem cells, monocytes and leukomonocytes). The separation and induction of EPCs can be achieved through many methods, including magnetic-activated cell sorting (Timmermans et al. 2007) and MNC adherent culture (Hill et al. 2003). EPCs separated by magnetic-activated cell sorting have consistent expression of EPC biomarkers, such as CD133, CD34 and FLK-1, but they proliferate much more slowly than EPCs separated by MNC adherent culture (Papathanasopoulos and Giannoudis 2008). CD34− population of cells in EPCs separated by MNC adherent culture, can secrete factors that stimulate CD34+ cell proliferation (Timmermans et al. 2007). EPCs separated by adherent culture comprise many small subpopulations that have no consistent cell surface markers and have been shown to have a higher proliferation capacity (Zampetaki et al. 2008). As magnetic-activated cell sorting is more expensive, we separated EPCs by the differential attachment method, which was used in early studies of EPCs (Jin et al. 2004). Here, we isolated MNCs from rat bone marrow using density gradient centrifugation, plated them in fibronectin-coated dishes, and cultured them under different conditions to investigate the biological characteristics of these differentiated MNCs.
We observed that MNCs in different culture media have different morphological phenotypes and exhibit different abilities to proliferate and adhere. The MNCs in CM, which were spindle-shaped and tended to form colonies, were different from MNCs in EGM-2MV, which were fusiform- or cobblestone-shaped and did not form colonies. The results suggested that stem cells and progenitor cells can adjust their morphologies to different microenvironments. Although the amount of serum in EGM-2MV is only half that of CM, EPCs proliferated more rapidly in EGM-2MV than in CM. There may be cytokines, such as vitamin C, rhEGF, IGF-1 and hydrocortisone, in EGM-2MV (but not in CM) that stimulate the EPCs to proliferate and differentiate. MNCs that expressed low levels of CD133 and FLK-1 were able to take up DiI-ac-LDL and bind lectin. It should be noted that, although the uptake of DiI-ac-LDL and the ability to bind lectin have previously been considered biomarkers of differentiating EPCs, recent research has demonstrated that monocytes and macrophage can also take up DiI-ac-LDL and bind lectin (Ouchi et al. 2001). In addition, we show that MNCs from rat bone marrow plated on Matrigel with EGM-2MV are able to form vascular-like structures in vitro, and western blotting results indicated that the MNCs induced by EGM-2MV expressed eNOS. Taken together, our results suggest that MNCs from rat bone marrow show biological characteristics of EPCs, such as a cobblestone-like morphology, DiI-ac-LDL-uptake, lectin-binding, continuous expression of eNOS, tube formation on Matrigel, and rapid replication from some cells.
EPCs have the stem cell characteristics of self-renewal and the capacity to differentiate into adult endothelial cells in vitro (Bessler 2004). Previous research has indicated that EPCs can be isolated from animal bone marrow (Zhang et al. 2006), peripheral blood (Park et al. 2003), umbilical cord blood (Ingram et al. 2004), and some organs (Zengin et al. 2006). It has been suggested that EPCs and hematopoietic stem cells are both derived from a common precursor, hemangioblasts (Murasawa and Asahara 2005); therefore, the markers of EPCs should have both stem cell and endothelial cell characteristics. At present, several surface markers, such as CD34, FLK-1 and CD133, have been generally accepted as biomarkers of EPCs (Yao et al. 2009), while CD31, vWF, and Factor VIII are used to identify mature endothelial cells. Friedrich et al. (2006) suggested that the CD34−/CD133+ EPC subpopulation is a precursor of CD34+/133+ EPCs. With the use of CD133 and FLK-1 as biomarkers to identify EPCs, we found that the percentages of CD133+ cells (9.51 ± 0.612%) and FLK-1+ cells (18.91 ± 3.17%) were not as high as Li et al. (2007) previously reported (CD133+ cells (36.08 ± 2.34%) and FLK-1+ cells (79.45 ± 3.12%)). The differences between these results might be explained by the use of different methods for detecting these biomarkers. Li et al. (2007) detected the surface markers of EPCs by immunohistochemistry and immunofluorescence, which can lead to false positives. We used FCM, which is more sensitive for detecting cell surface markers in a large population of cells. We further found that the peak expression of CD133 and FLK-1 occurred at different times in culture. The percentage of CD133+ cells was highest on day 12 (9.51 ± 0.612%), but the highest percentage of FLK-1+ (18.91 ± 3.17%) and FLK-1+/CD133+ cells (1.03 ± 0.16%) occurred on day 20 after seeding in fibronectin-coated dishes. However, the use of FLK-1/CD133 co-expression as a biomarker for identifying EPCs from rat bone marrow, peripheral blood and umbilical cord blood is still questionable.
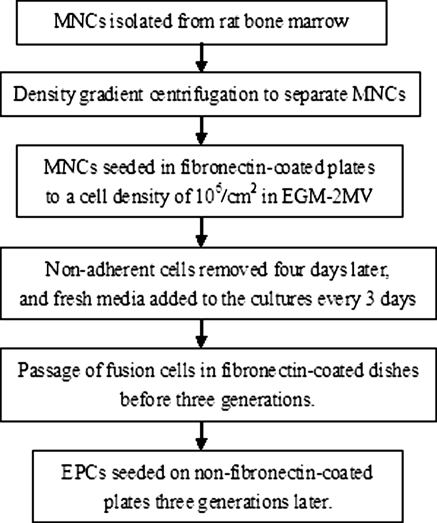
Previous reports have indicated that fibronectin combines with the transmembrane protein integrin to form a ligand-receptor complex that can not only mediate cell attachment, but can also promote cell proliferation, transference, differentiation, and even survival (Pagan et al. 2002). We also observed that fibronectin is required for the differentiation of MNCs to EPCs in the first three generations, which is consistent with previously reported results (Li et al. 2007), but fibronectin is not needed for the culture of EPCs after three generations. We found no significant differences in the percentage of CD133+ or CD133+/FLK-1+ cells, as assessed by FCM. However, EPCs grown on fibronectin-coated dishes showed lower FLK-1 expression and higher CD31 expression than EPCs grown on non-fibronectin-coated dishes. CD31 is the marker of differentiation of EPCs into endothelial cells, so we concluded that fibronectin at least partly contributed to the differentiation of EPCs into endothelial cells. Fibronectin is essential to induce the adhesion, proliferation and differentiation of MNCs to EPCs in the first three generations, while, to inhibit the differentiation of EPCs, culture without fibronectin may be an appropriate choice after three generations. In this study, we proposed to isolate and induce rat bone marrow-derived EPCs in accordance with the flow-chart format (Fig. 5).
Fig. 5.
The optimized procedure of rat bone marrow-derived EPCs isolation and culture
Jin et al. (2004) found that Early EPC with spindle shape showed peak growth at 2 to 3 weeks and died at 4 weeks, whereas late EPC with cobblestone shape appeared late at 2 to 3 weeks, showed exponential growth at 4 to 8 weeks, and lived up to 12 weeks. In this study, there were both spindle shape and cobblestone shape at 1 to 2 weeks. The two types of cells were isolated and cultured, respectively. FCM analysis of ECs specific markers showed that, in addition to FLK-1, the expression of CD133, CD31 and CD133/FLK-1 in cobblestone shape cells was slightly higher than in spindle shape cells (Table 1), with no significant difference. When the two types of cells were cultured continuously, the spindle shape cells disappeared and all cells showed cobblestone shape with the makers of ECs (Fig. 2). The two types of EPCs can both differentiate into ECs during long time of culture.
Angiogenesis and arteriogenesis are the two mechanisms of vasculogenesis (Buschmann and Schaper 1999). Some studies have shown that bone marrow-derived EPCs in peripheral blood participate in ischemic organization and the formation of neovasculature in tumor tissues (Takahashi et al. 1999), while other studies have suggested that bone marrow-derived EPCs do not significantly contribute to the formation of neovasculature in tumor tissues or to the repair of damaged vasculature (Hillebrands et al. 2002). For some diseases, such as vascular injury in atherosclerosis and ischemia, EPCs isolated from autologous bone marrow could be induced to differentiate in vitro and could be transplanted into the diseased lesion, which would eliminate the risk of rejection. However, in order to use autologous bone marrow transplants as a treatment, many technologies will need to be improved, including isolating a variety of subpopulations from bone marrow and inducing these subpopulations to differentiate into precise cell types. The experiments presented here, which have examined the biological characteristics of MNCs from rat bone marrow in different culture conditions, could provide the transplanted cells for disease therapy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 30971098) and the Province Natural Science Foundation of Shandong (Grant No. Z2008C03).
Abbreviations
- MNCs
Mononuclear cells
- EPCs
Endothelial progenitor cells
- eNOS
Endothelial nitric oxide synthase
- FCM
Flow cytometry
- ac-LDL
acetylated low density lipoprotein
References
- Asahara T, Murohara T, Sullivan A, Silver M, vanderZee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967 [DOI] [PubMed]
- Bessler M. Endothelial progenitor cells: reporting for duty. Blood. 2004;104:2616–2617. doi: 10.1182/blood-2004-08-3010. [DOI] [Google Scholar]
- Bradford GB, Williams B, Rossi R, Bertoncello I. Quiescence, cycling, and turn over on the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- Buschmann I, Schaper W. Arteriogenesis versus angiogenesis: two mechanisms of vessel growth. News Physiol Sci. 1999;14:121–125. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34–/CD133 + /VEGFR-2 + endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20–e25. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hillebrands JL, Klatter FA, Dijk WD, Rozing J. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med. 2002;8:194–195. doi: 10.1038/nm0302-194. [DOI] [PubMed] [Google Scholar]
- Igram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Jin H, Chang HY, Hyo SK, Jin HC, Hyun JK, Kyung KH, Byung HO, Myoung ML, Young BP. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- Li XQ, Meng QY, Wu HR. Effect of bone marrow-derived endothelial progenitor cell transplantation on vein microenvironment in a rat model of chronic thrombosis. Chin Med J. 2007;120:2245–2249. [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology. 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class a scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Pagan I, Khosla J, Li CM, Sannes PL. Effect of growth factor-fibronectin matrix interaction on rat type II cell adhesion and DNA sythesis. Exp Lung Res. 2002;28:69–84. doi: 10.1080/019021402753462013. [DOI] [PubMed] [Google Scholar]
- Papathanasopoulos A, Giannoudis PV (2008) Biological considerations of mesenchymal stem cells and endothelial progenitor cells. 5th Eur Symp Tissue Eng Bone Biol Congress 39:S21–S32 [DOI] [PubMed]
- Park A, Orosz K, Gupta S, Chambers R, Davidorf F, Moldovan N. Assessment of endothelial progenitor cells in the peripheral blood of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:B539–B564. [Google Scholar]
- Sandri M, Adams V, Gielen S, Linke A, Lenk K, Krankel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G, Hambrecht R. Effects of exercise and ischemiaon mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/8462. [DOI] [PubMed] [Google Scholar]
- Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B (2007) Endothelial outgrowth cells are not derived from CD133 cells or CD45 hematopoietic precursors Arterioscler Thromb Vasc Biol 27: 1572–1579 [DOI] [PubMed]
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- Yao WJ, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, Jamieson SW, Rubin LJ, Yuan JXJ. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L870–L878. doi: 10.1152/ajplung.90413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res. 2010;18:792–799. doi: 10.1038/cr.2008.69. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang GP, Jin HM, Hu RM. Characteristics of bone marrow-derived endothelial progenitor cells in aged mice. Biochem Biophys Res Commun. 2006;348:1018–1023. doi: 10.1016/j.bbrc.2006.07.161. [DOI] [PubMed] [Google Scholar]