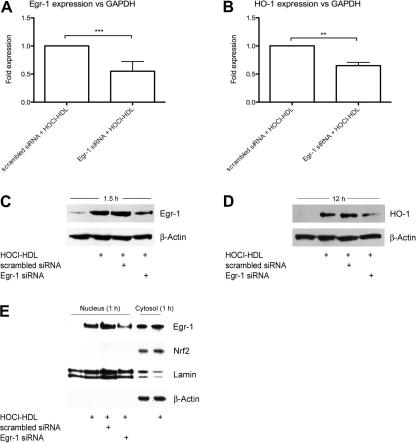
Fig. 8.
Real-time RT-PCR and Western blot analysis of HOCl–HDL-stimulated Egr-1 and HO-1 expression in endothelial cells treated with siRNA against Egr-1. (A/B) Cells were transfected with 50 nM siRNA against Egr-1 or with scrambled control siRNA. Forty-eight hours after transfection, cells were stimulated with 100 μg/ml HOCl–HDL for 1 h to determine Egr-1 mRNA expression (A) and for 4 h to assess HO-1 mRNA expression (B). Equal amounts of RNA were subjected to Real-time RT-PCR. Egr-1 and HO-1 expression were normalized to GAPDH (∗∗∗p < 0.001, ∗∗p < 0.01). (C and D) Cells were transfected with 50 nM siRNA against Egr-1 or with scrambled control siRNA and after 48 h cells were stimulated with 100 μg/ml HOCl–HDL for 1.5 h to assess Egr-1 (C) and for 12 h to determine HO-1 (D) protein expression. Cells were lysed and equal amounts of proteins were subjected to Western blot analysis. β-Actin was used as a loading control. Lane 1 represents non-stimulated controls, i.e. cells in the absence of lipoproteins. (E) Western blot analysis of HOCl–HDL-induced nuclear translocation of Egr-1 in cells treated with siRNA against Egr-1. Cells were transfected with 100 nM siRNA against Egr-1 or with scrambled control siRNA and after 48 h cells were stimulated with 100 μg/ml HOCl–HDL for 1 h. Cells were lysed, nuclear and cytosolic extracts were isolated and equal amounts of proteins were subjected to Western blot analysis for Egr-1 and HO-1. After stripping membranes, β-actin was used as a cytosolic and lamin as a nuclear loading control. Lanes 1 and 5 represents non-stimulated controls. (A–E) One representative experiment (performed in triplicate A/B) out of three is shown.