Abstract
BACKGROUND AND PURPOSE
The expression of voltage-dependent K+ channels (Kv) 1.5 is regulated by members of the heat shock protein (Hsp) family. We examined whether the heat shock transcription factor 1 (HSF-1) and its inducer geranylgeranylacetone (GGA) could affect the expression of Kv1.5 channels and its anchoring protein, synapse associated protein 97 (SAP97).
EXPERIMENTAL APPROACH
Transfected mouse atrial cardiomyocytes (HL-1 cells) and COS7 cells were subjected to luciferase reporter gene assay and whole-cell patch clamp. Protein and mRNA extracts were subjected to Western blot and quantitative real-time polymerase chain reaction.
KEY RESULTS
Heat shock of HL-1 cells induced expression of Hsp70, HSF-1, SAP97 and Kv1.5 proteins. These effects were reproduced by wild-type HSF-1. Both heat shock and expression of HSF-1, but not the R71G mutant, increased the SAP97 mRNA level. Small interfering RNA (siRNA) against SAP97 abolished HSF-1-induced increase of Kv1.5 and SAP97 proteins. A luciferase reporter gene assay revealed that the SAP97 promoter region (from −919 to −740) that contains heat shock elements (HSEs) was required for this induction. Suppression of SIRT1 function either by nicotinamide or siRNA decreased the level of SAP97 mRNA. SIRT1 activation by resveratrol had opposing effects. A treatment of the cells with GGA increased the level of SAP97 mRNA, Kv1.5 proteins and IKur current, which could be modified with either resveratrol or nicotinamide.
CONCLUSIONS AND IMPLICATIONS
HSF-1 induced transcription of SAP97 through SIRT1-dependent interaction with HSEs; the increase in SAP97 resulted in stabilization of Kv1.5 channels. These effects were mimicked by GGA.
Keywords: Kv1.5 channels, SAP97, HSF-1, GGA, SIRT1
Introduction
The Kv1.5 channel, a member of the superfamily of voltage-dependent K+ channels (Kv; channel nomenclature follows Alexander et al., 2009), carries the ultra-rapid component of the delayed-rectifier K+ channel current (IKur) in cardiomyocytes (Nattel et al., 1999). Because it plays an important role in action potential repolarization, it is of particular clinical importance as a target of antiarrhythmic drugs (Snyders et al., 1992).
The level of expression and proper distribution of Kv channels are crucial for determining the shape and duration of action potentials. PSD95/Dlg/ZO-1(PDZ) domain-containing proteins interact with various types of synaptic proteins, such as ion channels, signal transducers and cytoskeletal compartments, and regulate neural transmission (Garner et al., 2000). Especially, these proteins act as a scaffold to play a pivotal role in both organization and subcellular localization of Shaker-type K+ channels and N-methyl-D-aspartate (NMDA) receptors (Kim et al., 1995; Niethammer et al., 1996; Wyszynski et al., 1997). Membrane-associated guanylate kinase (MAGUK) proteins contain PDZ domains and are important factors for retention of channels in distinct domains of the plasma membrane, as described for retention of Kv4.2 channels in dendrite spines of hippocampal neurons (Gardoni et al., 2007). The synapse associated protein 97 (SAP97), one of the MAGUK family proteins, is known to be expressed in intercalated disks of myocardium where it colocalizes with Kv1.5 channels (Murata et al., 2001; Godreau et al., 2002). SAP97 is coimmunoprecipitated with Kv1.5 channels, suggesting a direct interaction between SAP97 and Kv1.5 proteins (Murata et al., 2001). It is assumed that SAP97 stabilizes Kv1.5 channels, by analogy to the neuronal MAGUK PSD95 protein that immobilizes the Kv1.4 channel and prevents its internalization (Reuver et al., 1998; Burke et al., 1999; Jugloff et al., 2000). Indeed, SAP97 binds to the three COOH-terminal amino acid residues of Kv1.5 channels, and coexpression of SAP97 and Kv1.5 channels increased IKur and 4-aminopyridine (4-AP) sensitive voltage-dependent currents in heterologously expressed cells (Murata et al., 2001; Abi-Char et al., 2007). These findings suggested that the reduced mobility of Kv subunits by anchoring proteins might be an important determinant of the proper assemblage of multiprotein complexes and the normal activation and regulation of potassium currents. Thus, it appears that SAP97 is one of the major regulators of Kv1.5 channel function.
Little is known about regulation of SAP97 expression. Growth factors and neurotrophins, such as brain-derived neurotrophic factor (BDNF) and neuregulin-1 (NRG1), have been reported to regulate SAP97 expression by altering its post-translational modifications (Xiong et al., 2002; Cotrufo et al., 2003; Jourdi et al., 2003). Recently, we reported that the heat shock factor 1 (HSF-1) inducer, geranylgeranylacetone (GGA), and heat shock protein (Hsp) 70 stabilize cardiac Kv1.5 proteins in the endoplasmic reticulum (ER) and Golgi apparatus to increase IKur (Hirota et al., 2008). HSF-1 is activated, trimerizes and accumulates in the nucleus. HSF-1 trimers bind with high affinity to HSEs that consist of multiple contiguous inverted repeats of the pentamer sequence nGAAn located in the promoter region of target genes (Westerheide and Morimoto, 2005). The purpose of the current study was to determine whether the transcription factor HSF-1 and its inducer GGA can affect expression of SAP97. We found that via binding of HSF-1 to heat shock elements (HSEs), HSF-1 and GGA could induce the transcription of SAP97. These effects were modified by a deacetylase and longevity factor, SIRT1.
Methods
Culture and transfection of HL-1 cardiomyocytes
A cardiac muscle cell line, HL-1, exhibits spontaneous contraction and retains phenotypic characteristics of adult cardiomyocytes (Claycomb et al., 1998). HL-1 cells were cultured in Claycomb medium (Sigma-Aldrich, Irvine, CA, USA) supplemented with 100 µM noradrenaline, 100 U·mL−1 penicillin, 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS, USA) and 4 mM L-glutamine (Wako, Osaka, Japan) in a humidified 5% CO2 incubator at 37°C. The medium was replaced every 24 h. The culture flasks were precoated overnight with Dulbecco's phosphate buffer saline (PBS) containing 0.012 mg·mL−1 fibronectin and 0.2 mg·mL−1 gelatin. For all overexpression experiments, cells on 35 mm dishes were transiently transfected using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). The transfection efficiency was estimated approximately 80% based on green fluorescent protein (GFP) fluorescence. Forty-eight hours after transfection, cells were harvested, and their extracted protein was subjected to Western blotting analysis. GGA, brefeldin A and colchicine were dissolved in dimethyl sulphoxide or ethanol. The final concentration of either dimethyl sulphoxide or ethanol in the culture medium was equal to or less than 0.01% v/v. GGA was kindly donated by Eisai Co., Tokyo, Japan.
Western blotting
Cells were lysed by sonication in a buffer (PBS/1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulphate, 10 µg·mL−1 aprotinin, 10 µg·mL−1 leupeptin, 10 µg·mL−1 pepstain and 1 mM phenylmethylsulphonyl-fluoride), and insoluble materials were removed by centrifugation (500 ×g, 10 min). Protein concentrations were determined by using NanoDrop A280 (Thermo Fisher Scientific Inc., Worcester, MA, USA); 2–10 µg of the protein was separated on 10% SDS-PAGE and electrotransferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were probed with antibodies against β-actin (1:5000, Calbiochem, La Jolla, CA, USA), Kv1.5 (1:200; Alomone Laboratories Ltd, Jerusalem, Israel), SAP97 (1:1000; Upstate, Billerica, MA, USA), Hsp70 (1:1000; Santa Cruz Bio., Santa Cruz, CA, USA) and HSF-1 (1:1000; provided from Professor Nakai) and were developed using an ECL Plus detection kit (Amersham Bio., Piscataway, NJ, USA).
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNAs of HL-1 cells were extracted using RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) and were subjected to an RT-PCR assay (Prime Script RT-PCR Kit; TAKARA Bio., Shiga, Japan). RNA samples were treated with DNase I (Promega Co., WI, USA) to eliminate genomic DNA. The PCR product was screened by 1% agarose gel electrophoresis, stained with ethidium bromide and visualized in a ultraviolet (UV) transilluminator. The primers are used for SAP97 (Dlg1): forward 5′-CAGGAAACGAGTGATGCTGA-3′, reverse 5′-GCTCGTCCCATACAGATGGT-3′; SIRT1: forward 5′-TTCCACTTTGGCATAAGGC-3′, reverse 5′-GCCATCCCTTCACGTTAG-3′; β-actin: forward 5′-CCAACCGCGAGAAGATGA-3′, reverse 5′-CCAGAGGCGTACAGGGATAG-3′.
Quantitative real-time PCR
Total RNA was extracted from cultured cells using RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. RNA samples were treated with DNaseI (Promega Co.) to eliminate genomic DNA contamination, and cDNA was synthesized using SuperScript™ II reverse transcriptase (Gibco-BRL, Grand Island, NY, USA). RT-PCR was done with the 7900HT Fast real-time RT-PCR System, 384-well plate according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). The universal ProbeLibrary (Roche, Basel, Switzerland) probes no. 93 and no. 63 were used for SAP97 and β-actin respectively. A SAP97 gene-specific primer set overlapping the middle of exon 19 and the middle of exon 20 (forward 5′-TTCCCGAAAATTTCCCTTCT-3′; reverse: 5′-TGGCATTAGAAGTTACGTGCTG-3′) was used to obtain 86 bp products. The primers for β-actin overlapped the end of exon 5 and the middle of exon 6 (forward 5′-GGATGCAGAAGGAGATTACTGC-3′; reverse 5′-CCACCGATCCACACAGAGTA-3′) resulting in 123 bp products. Data analysis was done with the SDS software version 3.2 (Applied Biosystems). cDNA was also amplified with PCR and separated by electrophoresis, stained with ethidium bromide and visualized in a UV transilluminator.
Construction of SAP97 promoter-luciferase fusion plasmids and luciferase reporter gene assay
A ∼1 Kb bp promoter fragment of mouse SAP97 gene was amplified by PCR using primers ([Nhe1 +1000 to +1026] and [HindIII −2000 to −1974]) (forward 5′-AGCTAGCGGAGGCTCTCTCCTTCCACCATGTG-3′) (reverse 5′-TGAACACGTGCTGGCCAACTTCAAGAAAAAGCTTAA-3′). The fragment contains putative HSEs (−919 to −714) predicted by Searching Protein and Nucleic Acid Sequence Motifs (MOTIF-SEARCH). It was ligated into the pGL3Basic Vector (Promega Co.) at NheI and HindIII (NEB BioLabs Inc., Cambridge, UK) sites (pGL3/SAP97) after filling-in reaction by Klenow reagent. A pGL3/ΔHSEs, deletion construct lacking the HSEs (−919 to −740) was generated. The DNA sequences were verified by the ABI PRISM® 7700 sequence detection system (Applied Biosystems). HL-1 cells on 35 mm dishes (1 × 105/well) were transfected for overnight with pGL3/SAP97, pGL3/ΔHSEs or the parental vector. pRL-TK vector (Renilla luciferase, Promega Co.) was used as an internal control of transfection efficiency. Luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega Co.) and analysed by a Wallac 1420 luminometer (Perkin Elmer Wallac Inc, Turku, Finland).
Knock down of SIRT1 and SAP97
Sequences of small interfering RNA (siRNA) oligonucleotides targeting the open reading frame of mouse mRNA are listed. SIRT1: sense 5′-CGAUGGACUCCUCACUAAUGG-3′; anti-sense 5′-AUUAGUGAGGAGUCCAUCGGU-3′ (Sigma-Aldrich); SAP97 siRNA target sequences: 5′-GCGGGUAAAUGACUGUAUA-3′ (Thermo Fisher Scientific Inc). HL-1 cells on 35 mm dishes were transfected overnight with siRNA oligonucleotides using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's instructions. Cells were lysed for 48 h after transfection, and extracts were subjected to Western blot analysis as described above.
Electrophysiological recordings
COS7 cells transfected with GFP and Kv1.5-FLAG plasmid were treated with GGA (Eisai Co.) at 100 nM for 12 h and subjected to the whole-cell patch clamp to measure the Kv1.5 channel-mediated membrane current. The pipette solution contained (in mM) 140 K aspartate, 5 MgCl2, 5 K2ATP, 5 EDTA, 5 HEPES (pH 7.2), and the external solution was Tyrode's solution. Procedures for the current measurement were essentially the same as described by Kato et al. (2005). Currents were elicited every 6 s by 500 ms test pulses ranging from −60 to +80 mV (in 10 mV increments). The holding potential was −60 mV. Kv1.5 current was measured as the difference between the currents consecutively recorded in the absence and presence of 1 mM 4-AP.
Densitometry analysis
Densitometry analysis was carried out using the digital imaging program Image J (U.S. National Institutes of Health). Each band was quantified by a mean pixel value after subtraction of background.
Statistical analysis
Data are presented as the mean ± SEM. Statistical significance was determined using Student's t-test. A P-value of <0.05 was considered statistically significant. All analyses were performed using StatView version 5.0.1 (SAS Institute Inc., Cary, NC, USA).
Materials
4-Aminopyridine (4-AP), brefeldin A and colchicine were purchased from Wako and noradrenaline, resveratrol and nicotinamide from Sigma-Aldrich.
Results
Heat shock and HSF-1 increased the protein levels of both SAP97 and Kv1.5 in HL-1 cells
Figure 1 shows the effect of heat shock at 42°C for 1 h on the protein levels of Hsp70, HSF-1, SAP97 and Kv1.5 channels in HL-1 cells. As expected, the protein levels of Hsp70 and HSF-1 significantly increased at 48 h after the treatment. Similarly, the protein levels of SAP97 and Kv1.5 channels were also significantly increased. We hypothesized that the increases of Kv1.5 and SAP97 proteins were attributable to the activation of HSF-1. To verify this hypothesis, we overexpressed HSF-1 and examined the levels of SAP97 and Kv1.5 proteins. As shown in Figure 2A, overexpression of HSF-1 significantly increased the protein levels of Kv1.5 and SAP97. In contrast, the HSF-1 mutant R71G, which lacks the ability to bind to HSEs, failed to increase the protein levels of Hsp70, SAP97 and Kv1.5 (Figure 2B), although R71G could react with the protein level of HSF-1 (Fujimoto et al., 2005).
Figure 1.
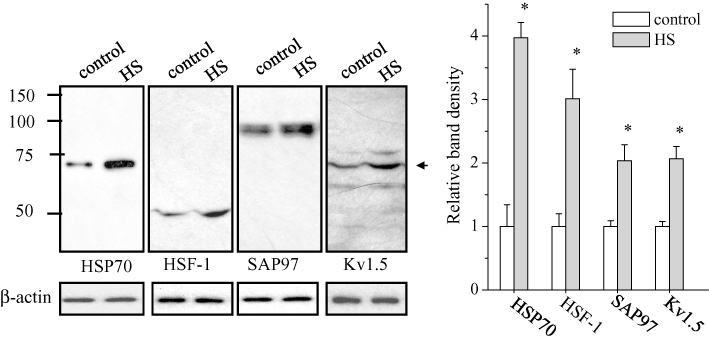
Heat shock (HS) regulation of the expression of HSP70, HSF-1, SAP97 and Kv1.5 proteins. HL-1 cells were treated with HS (at 42 °C for 1 h) and analysed by Western blotting using anti-HSP70, HSF-1, SAP97 and Kv1.5 antibodies (left). The blots at the bottom were reprobed with anti-β-actin as internal control. The mean ratio (±SEM) of each protein level normalized to β-actin (n = 6 for control and HS groups) is shown (right). Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05.
Figure 2.
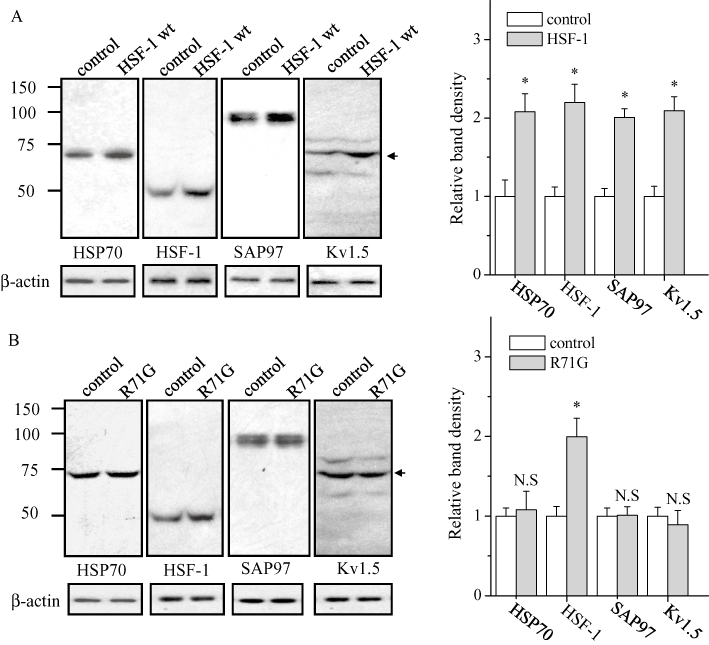
HSF-1 regulation of the expression of HSP70, HSF-1, SAP97 and Kv1.5 proteins. HL-1 cells were transfected with wild-type (Wt) HSF-1 (A) and mutant R71G (B) plasmids and analysed by Western blotting using anti-HSP70, HSF-1, SAP97 and Kv1.5 antibodies (left). The blots at the bottom were reprobed with anti-β-actin as internal control. The mean ratio (±SEM) of each protein level normalized to β-actin (n = 6 for control and transfected groups) is shown (right). Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05; N.S. = not statistically significant.
Heat shock and HSF-1 activated SAP97 transcription
To elucidate the mechanism of the HSF-1-induced increase in SAP97 protein, we examined the effects of heat shock and HSF-1 overexpression on the transcription of the SAP97 gene, in HL-1 cells using real-time PCR. Both heat shock at 42°C for 1 h and overexpression of wild-type HSF-1, but not the R71G mutant, significantly increased the level of SAP97 mRNA (Figure 3). MOTIF-SEARCH predicted the presence of HSEs from −919 to −740 of the SAP97 gene promoter region. To verify the function of these putative HSEs, we developed a pGL3 luciferase reporter gene construct that contains the ∼1 Kb promoter region (pGL3/SAP97) and a deletion construct that lacked the HSEs (pGL3/ΔHSEs). As shown in Figure 4, heat shock for 30 min at 42°C activated pGL3/SAP97 but not pGL3/ΔHSEs in HL-1 cells. Next, we tested whether HSF-1-induced activation of SAP97 altered the level of Kv1.5 channels. As shown in Figure 5, siRNA of SAP97 for overnight abolished the increase in Kv1.5 proteins induced by HSF-1, while scrambled siRNA did not influence the increase in Kv1.5 proteins by HSF-1.
Figure 3.
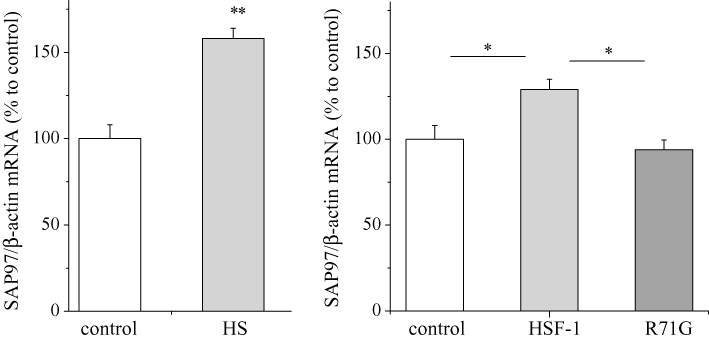
Effects of HS and HSF-1 overexpression on SAP97 mRNA expression. HL-1 cells were treated with HS (at 42°C for 1 h) (left), HSF-1 Wt and mutant R71G overexpression (right), then real-time PCR analysis were performed with SAP97 and β-actin primers. The mean ratio (±SEM) of each mRNA quantity normalized to β-actin (n = 6 for control and each treatment groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05, **P < 0.01. PCR, polymerase chain reaction.
Figure 4.
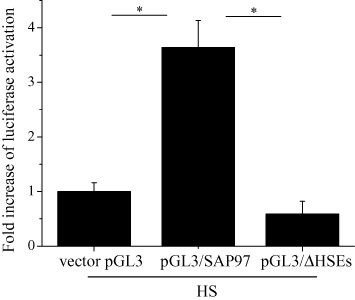
HS regulation of the activity of SAP97 promoter with HSEs. Effects of HS on luciferase activity in HL-1 cells transfected with reporter gene. Cells were co-transfected with SAP97 gene ∼1 Kb promoter-pGL3Basic luciferase reporter fusion plasmid containing (pGL3/SAP97) or lacking HSEs (pGL3/ΔHSEs) and pRL-TK vector as internal control; luciferase activities were measured after stimulation with HS (at 42°C for 30 min). The mean ratio (±SEM) of the firefly luciferase activity normalized to Renilla luciferase per well (n = 20 for pGL3 vector, pGL3/SAP97 and pGL3/ΔHSEs groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05. HSE, heat shock element.
Figure 5.
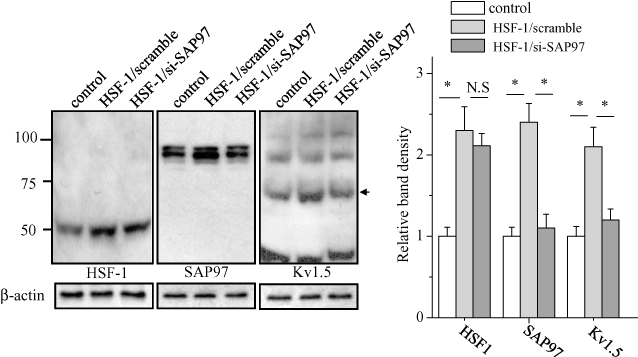
siRNA-SAP97 regulation in HSF-1-induced increases in expression of HSF-1, SAP97 and Kv1.5 proteins. HL-1 cells transfected with siRNA against SAP97 or scrambled siRNA were treated with Wt-HSF-1 plasmid and analyzed by Western blotting using anti-HSF-1, SAP97 and Kv1.5 antibodies. The blots at the bottom were reprobed with anti-β-actin as internal control. The mean ratio (±SEM) of each protein level normalized to β-actin (n = 3 for control and each treatment groups) is shown (right). Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05; N.S., not statistically significant; siRNA, small interfering RNA.
SIRT1 controls transcription of SAP97
It has been shown that acetylation of HSF-1 reduces its binding affinity to HSEs via recruitment of p300 (Westerheide et al., 2009). Moreover, activation of the deacetylase and longevity factor SIRT1 has been shown to prolong HSF-1 binding to HSEs (Westerheide and Morimoto, 2005). Thus, we examined whether modulators of SIRT1 function could influence the transcription of SAP97 in HL-1 cells transfected with HSF-1. An activator of SIRT1, resveratrol (50 µM), enhanced the effects of HSF-1 on SAP97 mRNA (Figure 6A), whereas an SIRT1 inhibitor, nicotinamide (5 mM), abolished its effects (Figure 6B). As expected, SIRT1 siRNA suppressed the heat shock–induced increase in SAP97 mRNA levels as shown in Figure 6C. SAP97 re-induced at the indicated recovery times after heat shock treatment despite knock down of SIRT1, suggesting heat shock may increase SAP97 mRNA through another pathway independent of SIRT1 regulation.
Figure 6.
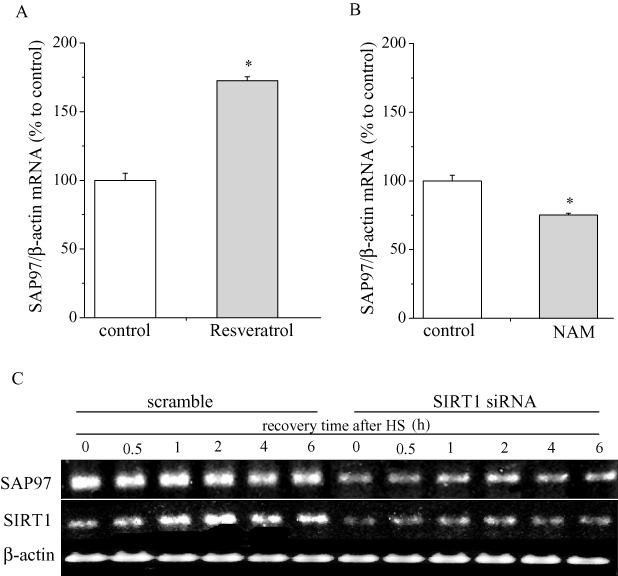
Effects of SIRT1 modulators on SAP97 transcription. (A) Effect of the SIRT1 inducer resveratrol on SAP97 mRNA expression. HL-1 cells transfected with HSF-1 were treated with resveratrol (50 µM) for overnight and real-time PCR analysis was performed with SAP97 and β-actin primers. The mean ratio (±SEM) of SAP97 mRNA quantity normalized to β-actin (n = 6 for control and resveratrol groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05. (B) Effect of the SIRT1 inhibitor nicotinamide (NAM) on SAP97 mRNA expression. HL-1 cells transfected with HSF-1 were treated with NAM (5 mM) overnight, and real-time PCR (RT-PCR) analysis was performed with SAP97 and β-actin primers. The mean ratio (±SEM) of SAP97 mRNA quantity normalized to β-actin (n = 6 for control and nicotinamide groups) is shown. Statistical significance of the differences between each group was tested by Student's t-test: *P < 0.05. C. Time courses of mRNA level of SAP97 and SIRT1 after HS with or without siRNA-SIRT1 inhibition of SAP97 transcription. HL-1 cells transfected with siRNA against SIRT1 or scrambled siRNA were treated at 42°C for 1 h and were harvested at indicated recovery time after HS. RT-PCR analysis was performed with SAP97, SIRT1 and β-actin primers. PCR. Polymerase chain reaction; siRNA, small interfering RNA.
GGA increased SAP97, Kv1.5 and IKur via activation of HSF-1
A nontoxic acyclic isoprenoid compound, GGA, has been reported to increase Hsp70 expression through activation of HSF-1 (Ooie et al., 2001). GGA increased HSF-1 in a concentration-dependent manner. The EC50 for GGA-induced increases of HSF-1 was 26.8 nM (h = 0.8), with peak effects at 100 nM (Figure 7).
Figure 7.
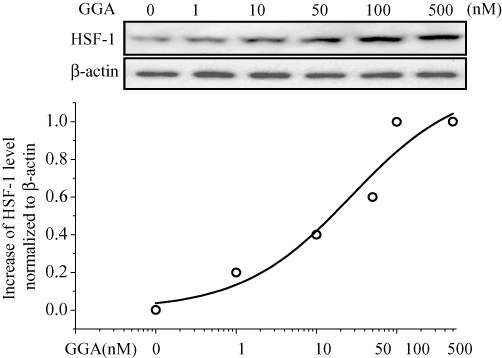
Dose-dependent effects of GGA on HSF-1 protein expression. HL-1 cells were treated with GGA at 0, 1, 10, 50, 100, 500 nM and analysed by Western blotting using anti-HSF-1 antibody. The blots at the bottom were reprobed with anti-β-actin as internal control (upper panel). The EC50 for GGA, on the expression of HSF-1, was 26.8 nM with h = 0.8 (n = 6). The values of relative band density of HSF-1 measured with various GGA concentrations were normalized to β-actin; their percentages were plotted as a function of GGA on a logarithmic scale. The line is a fit to the mean value of relative band density (n = 6), based on the following Hill equation: % relative band density = {(1 − S)·EC50h/(EC50h+[GGA]h) + S} × 100, which EC50 and h represent the half-maximum incremental concentration and Hill coefficient, respectively, with S standing for the residual component at higher GGA (lower panel). GGA, geranylgeranylacetone.
GGA at 100 nM significantly increased the protein levels of Kv1.5 channels and SAP97 as well as SAP97 mRNA (Figure 8A,B). It increased the luciferase activity in HL-1 cells transfected with pGL3/SAP97 but not in cells transfected with pGL3/ΔHSEs (Figure 8C), indicating that GGA increased SAP97 mRNA through an interaction between HSF-1 and the HSEs in the SAP97 promoter region. As shown in Figure 9, resveratrol enhanced the effects of GGA on SAP97 mRNA, and again, nicotinamide abolished its effect.
Figure 8.
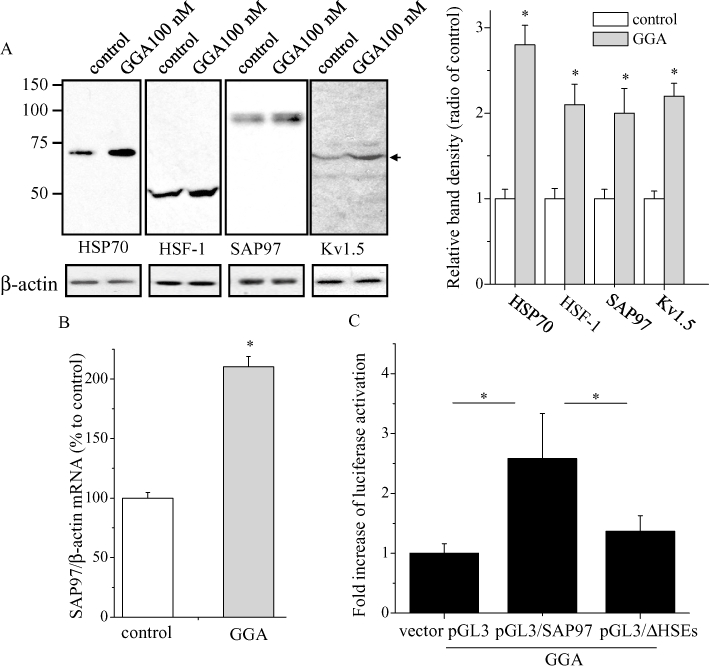
Effects of GGA on the regulations of SAP97 and related proteins. (A) GGA (100 nM) regulates expression of HSP70, HSF-1, SAP97 and Kv1.5 proteins. HL-1 cells were treated with GGA 100 nM or ethanol overnight and analyzed by Western blotting using anti-Hsp70, HSF-1, SAP97 and Kv1.5 antibodies. The blots at the bottom were reprobed with anti-β-actin as internal control. The mean ratio (±SEM) of each protein level normalized to β-actin (n = 6 for control and GGA groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05. (B) Effect of GGA 100 nM on SAP97 mRNA expression. HL-1 cells were treated with GGA 100 nM for overnight, and real-time PCR analysis was performed with SAP97 and β-actin primers. The mean ratio (±SEM) of SAP97 mRNA quantity normalized to β-actin (n = 6 for control and GGA groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05. (C) GGA 100 nM regulation of the activity of SAP97 promoter with HSEs. Effects of GGA on luciferase activity in HL-1 cells transfected with reporter gene. Cells were cotransfected with SAP97 gene ∼1 Kb promoter-pGL3 basic luciferase reporter fusion plasmid containing (pGL3/SAP97) or lacking HSEs (pGL3/ΔHSEs) and the pRL-TK vector as internal control; luciferase activities were measured after stimulation with GGA 100 nM. The mean ratio (±SEM) of firefly luciferase activity normalized to Renilla luciferase per well (n = 20 for pGL3 vector, pGL3/SAP97 and pGL3/ΔHSEs groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05. GGA, geranylgeranylacetone; HSE, heat shock element.
Figure 9.
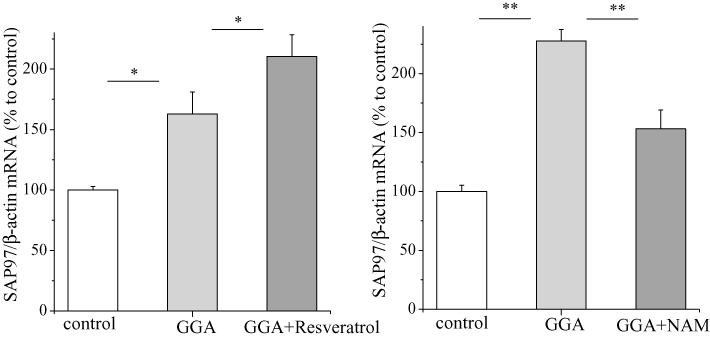
Effects of SIRT1 modulators in GGA-induced increase on SAP97 mRNA. HL-1 cells treated with the SIRT1 activator resveratol (50 µM), in (A) or the SIRT1 inhibitor nicotinamide (5 mM) overnight, in (B) after stimulation with GGA (100 nM) overnight, and real-time PCR analysis was performed with SAP97 and β-actin primers. The mean ratio (±SEM) of SAP97 mRNA quantity normalized to β-actin (n = 6 for control and each treatment groups) is shown. Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05, **P < 0.01. GGA, geranylgeranylacetone; PCR, polymerase chain reaction.
GGA increased IKur currents through cell surface Kv1.5 channels
We further examined effects of GGA on IKur in cells expressing Kv1.5 channels. In COS7 cells expressing Kv1.5-FLAG proteins, GGA treatment at 100 nM for 12 h significantly increased IKur at test potentials of +20 to +80 mV (Figure 10A,B). This effect required preincubation of the cells with the drug, as GGA had no effect when applied immediately before the current recording (data not shown). Brefeldin A inhibits ADP ribosylation factor–dependent protein transport in the Golgi apparatus, whereas colchicine inhibits microtubule-dependent protein transport (Kato et al., 2005). To substantiate the hypothesis that GGA-induced increase of IKur was due to stabilization of Kv1.5 channels, we tested effects of brefeldin A and colchicine (both at 5 µM for 12 h) on IKur. Treatment with either drug decreased the basal level of IKur and abolished the GGA-induced increase in IKur (Figure 10C). These results indicated that GGA-induced increase of IKur was caused by stabilization of this protein.
Figure 10.
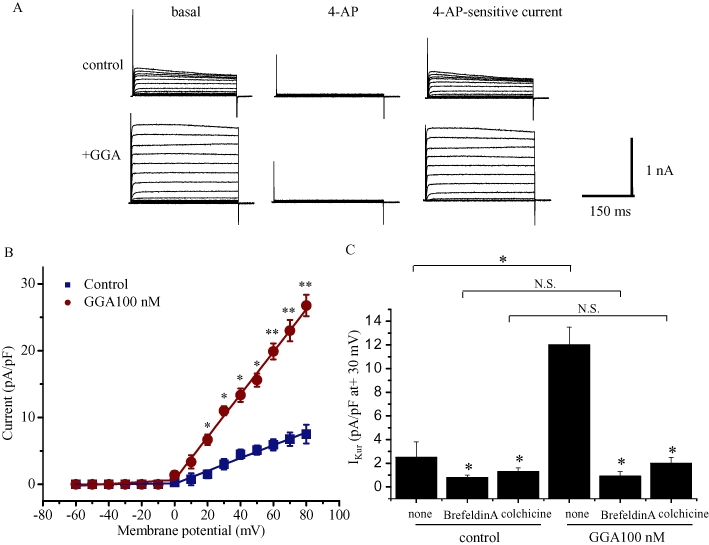
Effects of GGA on IKur in COS7 cells transfected with Kv1.5-FLAG channels. (A) Representative current traces from COS7 cells treated with GGA 100 nM or vehicle control (0.01% ethanol) for 12 h. The currents were elicited by 500 ms test pulses ranging from −60 to +80 mV (in 10 mV increments). The holding potential was −60 mV. Right panels show the 4-AP sensitive currents obtained by subtracting the currents recorded in the presence of 1 mM 4-AP (middle) from those recorded in the absence of 4-AP (left). (B) Current–voltage relationships of 4-AP-sensitive currents in the presence and absence of GGA. The peak amplitudes of the currents are presented as the mean ratio (±SEM) (pA/pF) (n = 21 for control and GGA 100 nM groups). Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05, **P < 0.01. C. Effect of brefeldin A and colchicine on GGA 100 nM-induced increases in IKur currents elicited by 500 ms test pulse to +30 mV. Cells were treated with brefeldin A or colchicine (both at 5 µM for 12 h) before determination of IKur currents in the presence of GGA 100 nM (n = 12 for control and each treatment groups). Statistical significance of the differences among each group was tested by Student's t-test: *P < 0.05, N.S. = not statistically significant. GGA, geranylgeranylacetone.
Discussion and conclusion
IKur and the Ca2+-independent transient outward currents (Ito) are among the main repolarizing currents that contribute to the early phase of atrial and ventricular repolarization. There is a general consensus that the voltage-dependent Kv1.5 and Kv4.2/4.3 channels are the main molecular determinants of atrial IKur and ventricular Ito respectively. SAP97 is abundantly expressed in myocardium and is known to regulate the formation of channel complexes by anchoring Kv channel subunits on the plasma membrane. SAP97 colocalizes and coprecipitated with Kv1.5 channel proteins in cardiac myocytes and enhances currents carried by Kv1.5 channels (Murata et al., 2001). SAP97 can also interact with the inward rectifier K+ channels via its PDZ domains and makes the G protein–sensitive current (Hibino et al., 2000). Ito is a major repolarizing K+ current in ventricular myocytes, which contributes to the early phase of repolarization, influencing the shape and duration of action potential. SAP97 has also been reported to interact with Kv4.2/4.3 channels to regulate Ito currents (El-Haou et al., 2009).
As a linker protein, SAP97 connects signaling proteins and their targets. Phosphorylation of β-adrenoceptors by cAMP-dependent protein kinase depends on SAP97/AKAP interaction (Gardner et al., 2007). SAP97 works as a molecular adaptor between Kv1.4 channels and the calmodulin-dependent protein kinase (CaMK)II as well (Kim et al., 1996). Taken together, SAP97 creates the scaffold complex that anchors several channels in the plasma membrane. Therefore, the up-regulation of SAP97, through activation of HSF-1, could activate both IKur and Ito in the cardiac myocytes and modify responses to adrenergic signaling in the heart, influencing the shape and duration of action potentials in both atrial and ventricular myocytes.
The growth factors and neurotrophins, such as BDNF and NRG-1, have been reported to regulate postsynaptic density protein 95/cysteine-rich intestinal protein (PSD-95/Crip), though these growth factors increased SAP97 protein expression without changes in its mRNA, indicating their translational or post-translational modification of SAP97 protein (Xiong et al., 2002; Cotrufo et al., 2003; Jourdi et al., 2003). In our study, we demonstrated that heat shock increased SAP97 mRNA via activation of HSF-1. This increase in the protein level of SAP97 was associated with stabilization of Kv1.5 channels in HL-1 cells. This is the first report to demonstrate that heat shock and HSF-1 could directly up-regulate SAP97 mRNA levels via binding to HSEs. Thus, the SAP97 gene could be a novel class of genes regulated by HSF-1.
It is well known that HSF-1 is post-translationally modified by phosphorylation and modification of SUMO-1 to achieve a further dynamic range of action (Hong et al., 2001). Binding of HSF-1 to its target promoter sites has recently been reported to be regulated by either acetylase or deacetylase action (Rabindran et al., 1994). SIRT1 directly deacetylates HSF-1 and thereby regulates the heat shock response in mammalian cells (Westerheide et al., 2009). In the present study, a SIRT1 activator, resveratrol, increased the mRNA of SAP97 and a SIRT1 inhibitor, nicotinamide, decreased it, findings confirmed by a knock-down experiment of SIRT1 using siRNA abolished the heat shock–induced increase in SAP97 mRNA level. These data indicated an involvement of SIRT1 in HSF-1-induced activation of SAP97 transcription.
During atrial fibrillation or atrial dilation, the density of IKur has been reported to be unaltered or only slightly decreased despite the decrease in Kv1.5 channels in the atrial myocardium (Le Grand et al., 1994; Van Wagoner et al., 1997; Workman et al., 2001). Abi-Char et al. (2007) suggested a compensating response for the down-regulation of Kv1.5 channels and up-regulation of SAP97 through activation of HSF-1 might be one of the cellular responses to compensate for the down-regulation of Kv1.5 channels. Indeed, SAP97 stabilized cardiac Kv1.5 channels (Murata et al., 2001), which is consistent with our results. Cardiac hypertrophy is associated with reduction of both Kv4.1/4.2 proteins and repolarizing K+ currents (Ito) that leads to an increased APD and electrical vulnerability (Oudit et al., 2001). Thus, HSF-1-induced up-regulation of SAP97 also may be important to compensate for the down-regulation of Kv4.1/4.2 channel activity in these conditions.
A regulator of HSF-1 could be of benefit for treatment of atrial fibrillation or ventricular arrhythmia in cardiac hypertrophy that reduces the stability of Kv1.5 as well as Kv4.2/4.3 channels. GGA is a nontoxic acyclic isoprenoid compound with a retinoid skeleton that could increase Hsp70 expression through activation of HSF-1 in various tissues (Ooie et al., 2001). In HL-1 cells, GGA prevents atrial remodelling induced by tachypacing and oral administration of GGA protects atrial fibrillation–induced atrial remodeling (Brundel et al., 2006a,b;). In the present study, we report for the first time that GGA can (i) up-regulate the SAP97 mRNA via HSEs activation, (ii) increase SAP97 protein levels, (iii) stabilize Kv1.5 channels and (iv) enhance IKur.
The increase of IKur by GGA may shorten the APD of atrial myocytes, especially in chronic atrial fibrillation. As Kv1.5 channels are reduced as a consequence of mutations that cause hereditary human atrial fibrillation to induce triggered activity, GGA-induced increases of SAP97 and Kv1.5 channels might be beneficial under such pathological condition. However, its benefit might be restricted to specific mutation. On the contrary, GGA-induced activation of IKur or Ito through the activation of transcription of SAP97 might be restricted to specific mutations and shorten the atrial and ventricular APD. Since it is well established that activation of Kv1.5 channel currents shortens an effective refractory period, GGA-induced activation of IKur or Ito may lead to atrial fibrillation as well as ventricular arrhythmia.
It has been reported that SIRT1 works as an endogenous inhibitor of stress-mediated cell death in the cardiac myocytes as shown by the prevention of the cardiac cellular apoptosis by resveratrol (Ancendor et al., 2004). Cellular apoptosis is well known to be caused by elevation of intracellular Ca2+ concentration. Activation of SIRT1 by resveratrol could augment the effects of GGA on SAP97 transcription to activate IKur. As a result, the shortening of the APD could decrease the cardiac intracellular Ca2+ concentration to inhibit mitochondrial Ca2+ uptake, mitochondrial permeation transport pore and Bax protein (Chen et al., 2005). Resveratrol-induced activation of SIRT1 may also be involved in the antiapoptotic effect of this drug. SIRT1 might be a therapeutic target to enhance the beneficial effects of GGA to prevent cardiac apoptosis.
Acknowledgments
We thank Dr WC Claycomb for providing HL-1 cardiac myocytes as a kind gift. GGA was kindly donated by Eisai Co., Japan.
Glossary
Abbreviations
- 4-AP
4-aminopyridine
- APD
action potential duration
- BDNF
brain-derived neurotrophic factor
- ER
endoplasmic reticulum
- GGA
geranylgeranylacetone
- HSEs
heat shock elements
- HSF-1
heat shock factor 1
- Hsp
heat shock protein
- IKur
the ultra-rapid component of the delayed-rectifier K+ channel current
- MAGUK
membrane associated guanylate kinase
- NMDA
N-methyl-D-aspartate
- NRG1
neuregulin-1
- PDZ
PSD95/Dlg/ZO-1 proteins
- PSD-95/Crip
postsynaptic density protein 95/ cysteine-rich intestinal protein
- SAP97
synapse associated protein 97
Conflicts of interest
None.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Abi-Char J, Maguy A, Coulombe A, Balse E, Ratajczak P, Samuel JL, et al. Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes. J Physiol. 2007;582:1205–1217. doi: 10.1113/jphysiol.2007.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158:S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancendor RR, Kirshenbaum LA, Imai S-I, Sadoshima J. Silent information regulator 2a, a longevity factor and a class III histone deacetylase, is an essential endogenous inhibitor of apoptosis in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Henning RH, Ke L, van Gelder IC, Crijns HJ, Kampinga HH. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J Mol Cell Cardiol. 2006a;41:555–562. doi: 10.1016/j.yjmcc.2006.06.068. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Shiroshita-Takeshita A, Qi X, Yeh YH, Chartier D, van Gelder IC, et al. Induction of heat shock response protects the heart against atrial fibrillation. Circ Res. 2006b;99:1394–1402. doi: 10.1161/01.RES.0000252323.83137.fe. [DOI] [PubMed] [Google Scholar]
- Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol. 1999;113:71–80. doi: 10.1085/jgp.113.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Haou S, Balse E, Neyroud N, Dilanian G, Gavillet B, Abriel H, et al. Kv4 potassium channels form a tripartite complex with the anchoring protein SAP97 and CAMKII in cardiac myocytes. Cir Res. 2009;104:758–769. doi: 10.1161/CIRCRESAHA.108.191007. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Hayashi T, Kitaura Y, Tanaka Y, Inouye S, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282:5085–5099. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Mauceri D, Marcello E, Sala C, Di Luca M, Jeromin A. SAP97 directs the localization of Kv4.2 to spines in hippocampal neurons: regulation by CAMKII. J Biol Chem. 2007;282:28691–28699. doi: 10.1074/jbc.M701899200. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Godreau D, Vranckx R, Maguy A, Rücker-Martin C, Goyenvalle C, Abdelshafy S, et al. Expression, regulation and role of the MAGUK protein SAP-97 in human atrial myocardium. Cardiovasc. 2002;56:433–442. doi: 10.1016/s0008-6363(02)00602-8. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Tanemoto M, Fujita A, Doi K, Kubo T, et al. Anchoring proteins confer G protein sensitive to an inward-rectifier K(+) channel through the GH domain. EMBO J. 2000;19:78–83. doi: 10.1093/emboj/19.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Kurata Y, Kato M, Notsu T, Koshida S, Inoue T, et al. Functional stabilization of Kv1.5 protein by Hsp70 in mammalian cell lines. Biochem Biophys Res Commun. 2008;372:469–474. doi: 10.1016/j.bbrc.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Hong Y, Rogers R, Matunis MJ, Mayhew CN, Goodson ML, Park-Sarge OK, et al. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J Biol Chem. 2001;276:40263–40267. doi: 10.1074/jbc.M104714200. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, et al. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff DG, Khanna R, Schlichter LC, Jones OT. Internalization of the Kv1.4 potassium channel is suppressed by clustering interactions with PSD-95. J Biol Chem. 2000;275:1357–1364. doi: 10.1074/jbc.275.2.1357. [DOI] [PubMed] [Google Scholar]
- Kato M, Ogura K, Miake J, Sasaki N, Taniguchi S, Igawa O, et al. Evidence for proteasomal degradation of Kv1.5 channel protein. Biochem Biophys Res Commun. 2005;337:343–348. doi: 10.1016/j.bbrc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated Putative Guanylate Kinases. Nuropharmacol. 1996;35:993–1000. doi: 10.1016/0028-3908(96)00093-7. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Le Grand BL, Hatem S, Deroubaix E, Couétil JP, Coraboeuf E. Depressed transient outward and calcium currents in dilated human atria. Cardiovasc. 1994;28:548–556. doi: 10.1093/cvr/28.4.548. [DOI] [PubMed] [Google Scholar]
- Murata M, Buckett PD, Zhou J, Brunner M, Folco E, Koren G. SAP97 interacts with Kv1.5 in heterologous expression systems. Am J Physiol Heart Circ Physiol. 2001;281:2575–2584. doi: 10.1152/ajpheart.2001.281.6.H2575. [DOI] [PubMed] [Google Scholar]
- Nattel S, Yue L, Wang Z. Cardiac ultrarapid delayed rectifiers: a novel potassium current family o f functional similarity and molecular diversity. Cell Physiol Biochem. 1999;9:217–226. doi: 10.1159/000016318. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, et al. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Bachx PH. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Wisniewski J, Li L, Li GC, Wu C. Interaction between heat shock factor and hsp70 is insufficient to suppress induction of DNA-binding activity in vivo. Mol Cell Biol. 1994;14:6552–6560. doi: 10.1128/mcb.14.10.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- Snyders J, Knoth KM, Roberds SL, Tamkun MM. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol. 1992;41:322–330. [PubMed] [Google Scholar]
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, et al. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- Xiong H, Futamura T, Jourdi H, Zhou H, Takei N, Diverse-Pierluissi M, et al. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology. 2002;42:903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


