Figure 3.
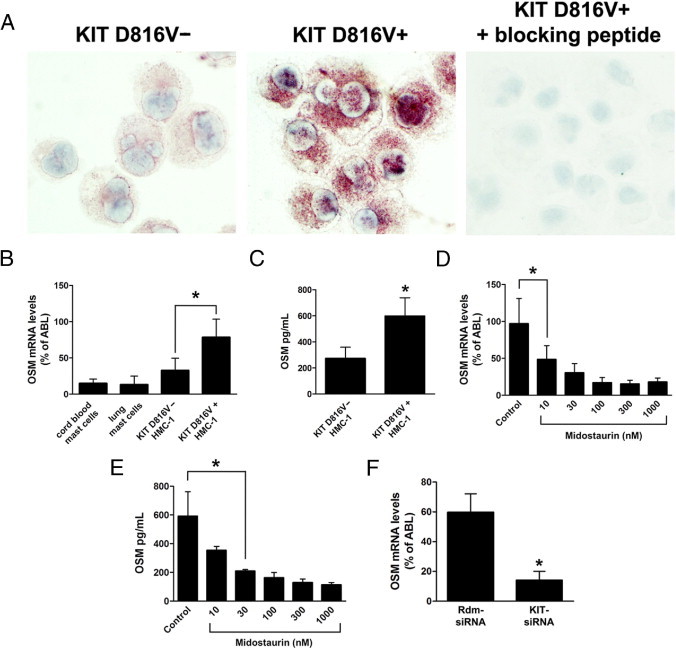
Expression of OSM in the HMC-1 mast cell line. A:KIT D816V− and KIT D816V+ HMC-1 cells were spun onto cytospin slides and expression of OSM was analyzed by immunocytochemistry using an anti-OSM antibody. Preincubation of the anti-OSM antibody with a specific blocking peptide abolished OSM immunoreactivity. B: Cord blood-derived mast cells, primary human lung mast cells, KIT D816V− HMC-1 cells, and KIT D816V+ HMC-1 cells were analyzed for expression of OSM by quantitative real-time PCR. C:KIT D816V− HMC-1 and KIT D816V+ HMC-1 cells were incubated at a density of 4 × 106 cells/mL for 24 hours. Supernatants were then harvested, and the amount of secreted OSM was determined by ELISA. D and E:KIT D816V+ HMC-1 cells (4 × 106 cells/mL) were treated with midostaurin at concentrations from 10 nmol/L to 1000 nmol/L for 24 hours. Cells and supernatants were then harvested, and expression of OSM mRNA (D) and of secreted OSM protein (E) was determined. F:KIT D816V+ HMC-1 cells were lentivirally transduced with a small interfering RNA targeting KIT (KIT-siRNA) or with a random sequence siRNA (Rdm-siRNA). At 24 hours after transduction, cells were selected with puromycin (2 μg/mL) for 48 hours. Expression of OSM was then determined by quantitative real-time PCR. All results represent the mean ± SD of three independent experiments. *P < 0.05.
