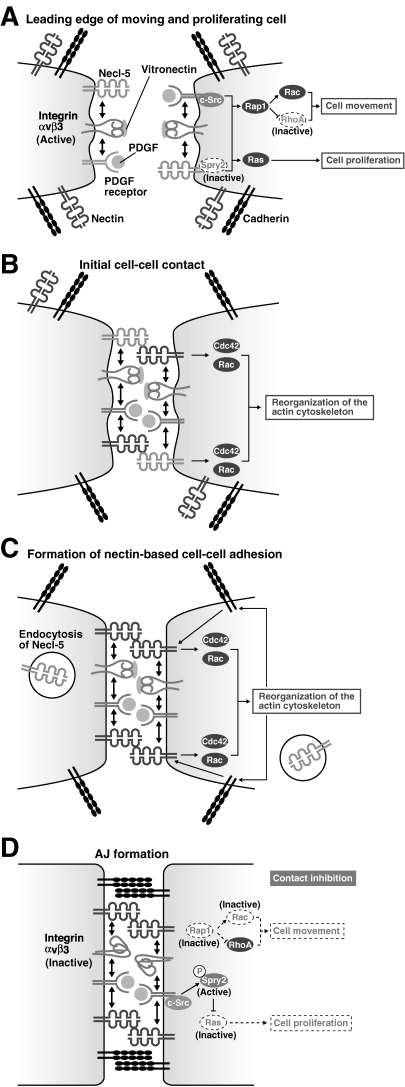
Figure 4.
Regulation of cell movement and proliferation by nectins and Necl-5. (A) Necl-5, integrin αvβ3 and PDGF receptor form a complex at the leading edge of a moving cell and enhance cell movement by inducing c-Src and Rap1 activation, which leads to the activation of Rac together with the inhibition of RhoA. The ternary complex also enhances cell proliferation by inducing the activation of the Ras-mediated signalling pathway through inhibiting Necl-5-associated sprouty2 (Spry2). At this stage, nectins and cadherins are sparsely distributed on the cell surface. (B) The initial cell–cell contact is formed by the trans-interaction of nectin-3 with Necl-5. At this stage, the reorganization of the actin cytoskeleton starts with the activation of Cdc42 and Rac. (C) The trans-interaction of nectin-3 with Necl-5 is transient, and Necl-5 is subsequently downregulated from the cell surface by endocytosis. Next, trans-interaction of nectins occurs. (D) Cadherins are recruited to nectin-based cell–cell adhesion sites and homophilically interact in trans to form adherens junctions (AJs). At this stage, integrin αvβ3 becomes inactive by the action of trans-interacting nectins. Due to the downregulation of Necl-5 from the cell surface, Spry2 is released from Necl-5 and is tyrosine-phosphorylated by c-Src and becomes active to inhibit the Ras-mediated cell proliferation signals. The intracellular signalling mediated by integrin αvβ3 and PDGF receptor is then suppressed, resulting in the inhibition of cell movement and proliferation (contact inhibition).