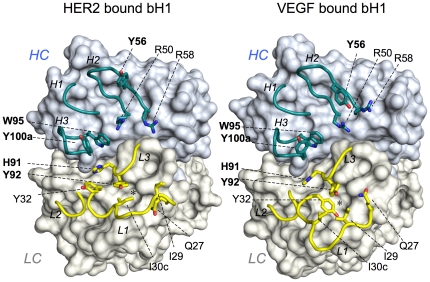
Figure 1. The distinct CDR conformations of bH1.
The three HC CDRs (cyan) and three LC CDRs (yellow) of bH1 in complex with HER2 (PDB code 3BE1) or VEGF (PDB code 3BDY) are shown in cartoon representation with the rest of bH1 structure as surface. Selected residues are shown in stick representation: LC-I29, Y32 and I30c are specifically important for bH1-44/VEGF binding and HC-R50 and R58 are specifically important for bH1-44/HER2 binding whereas LC-H91 and Y92, and HC-Y56, W95 and Y100a (in bold) are residues important for the bH1-44 interaction with both antigens as well as the Herceptin interaction with HER2. Note the highly distinct conformations of CDR-L1, the adjustment of the side chains of highlighted residues, and the side chains of LC-I30c and LC-Y32 that alternatively occupy the nearby cavity (*) in HER2 bound bH1 or VEGF bound bH1, respectively. See Figure S1 movie (Movie S1) morphing the two structures, which highlights the extent of the conformational adjustment for dual interaction.